Pharmacokinetics and Comparative Bioavailability of a Levothyroxine Sodium Oral Solution and Soft Capsule
- PMID: 30153382
- PMCID: PMC6585626
- DOI: 10.1002/cpdd.608
Pharmacokinetics and Comparative Bioavailability of a Levothyroxine Sodium Oral Solution and Soft Capsule
Abstract
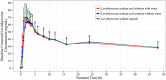
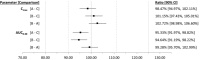
A new formulation of levothyroxine sodium has been developed in the form of an oral solution contained in unit-dose ampules. A study has been conducted to compare the bioavailability of levothyroxine sodium oral solution and levothyroxine sodium soft capsule in healthy volunteers under fasting conditions. The rate and extent of absorption of the new levothyroxine solution were also evaluated when administered on dilution in water or directly into the mouth without water. In each period, according to the randomization scheme, subjects were administered single oral doses of either test, as 4 × 150-μg unit-dose ampules, with or without water, or reference, as 4 × 150-μg capsules in a crossover design. Thirty-six subjects were randomized and dosed in this study; of these, 31 completed all study periods. When comparing the solution with the capsule (both products administered with water), the 90% confidence intervals for the ratio of log-transformed values of AUC0-48 and Cmax were within 90.00% and 111.11%, respectively, for baseline-corrected levothyroxine. Moreover, the administration of levothyroxine oral solution without water did not affect the rate and extent of its absorption. In conclusion, levothyroxine oral solution unit-dose ampules were bioequivalent to the levothyroxine capsule when administered with or without water. All formulations were well tolerated, with no major side effects.
Keywords: bioavailability; bioequivalence; levothyroxine; oral solution; pharmacokinetics.
© 2018 The Authors. Clinical Pharmacology in Drug Development Published by Wiley Periodicals, Inc. on behalf of The American College of Clinical Pharmacology.
Figures
References
-
- Wonsick C, Hays MT. Absorption of oral thyroxine. Endocrinologist, 1995;5:222–228.
-
- Hays MT. Thyroid hormone and the gut. Endocr Res. 1988;14:203–224. - PubMed
-
- Hays MT. Localization of human thyroid absorption. Thyroid. 1991;1:241–248. - PubMed
-
- Wenzel KW, Kirschsieper HE. Aspects of the absorption of oral L‐thyroxine in normal man. Metabolism. 1977;26:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

