Lineage-specific exosomes could override extracellular matrix mediated human mesenchymal stem cell differentiation
- PMID: 30153612
- PMCID: PMC6371403
- DOI: 10.1016/j.biomaterials.2018.08.027
Lineage-specific exosomes could override extracellular matrix mediated human mesenchymal stem cell differentiation
Abstract
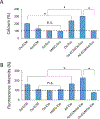
Lineage specification is an essential process in stem cell fate, tissue homeostasis and development. Microenvironmental cues provide direct and selective extrinsic signals to regulate lineage specification of stem cells. Microenvironmental milieu consists of two essential components, one being extracellular matrix (ECM) as the substratum, while the other being cell secreted exosomes and growth factors. ECM of differentiated cells modulates phenotypic expression of stem cells, while their exosomes contain phenotype specific instructive factors (miRNA, RNA and proteins) that control stem cell differentiation. This study demonstrates that osteoblasts-derived (Os-Exo) and adipocytes-derived (Ad-Exo) exosomes contain instructive factors that regulate the lineage specification of human mesenchymal stem cells (hMSCs). Analyses of exosomes revealed the presence of transcription factors in the form of RNA and protein for osteoblasts (RUNX2 and OSX) and adipocytes (C/EBPα and PPARγ). In addition, several miRNAs reported to have osteogenic and adipogenic differentiation potentials are also identified in these exosomes. Kinetic and differentiation analyses indicate that both osteoblast and adipocyte exosomes augment ECM-mediated differentiation of hMSCs into the respective lineage. The combination of osteoblast/adipocyte ECM and exosomes turned-on the lineage specific gene expressions at earlier time points of differentiation compared to the respective ECM or exosomes administered individually. Interestingly, the hMSCs differentiated on osteoblast ECM with adipogenic exosomes showed expression of adipogenic lineage genes, while hMSCs differentiated on adipocyte ECM with osteoblast exosomes showed osteogenic lineage genes. Based on these observations, we conclude that exosomes might override the ECM mediated instructive signals during lineage specification of hMSC.
Keywords: Adipogenesis; Exosomes; Extracellular matrix; Lineage determination; Microenvironment; Osteogenesis; Stem cell fate.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
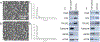

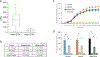
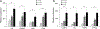



Similar articles
-
Syndecan-1 Facilitates the Human Mesenchymal Stem Cell Osteo-Adipogenic Balance.Int J Mol Sci. 2020 May 29;21(11):3884. doi: 10.3390/ijms21113884. Int J Mol Sci. 2020. PMID: 32485953 Free PMC article.
-
Epigenetic Plasticity Drives Adipogenic and Osteogenic Differentiation of Marrow-derived Mesenchymal Stem Cells.J Biol Chem. 2016 Aug 19;291(34):17829-47. doi: 10.1074/jbc.M116.736538. Epub 2016 Jul 11. J Biol Chem. 2016. PMID: 27402842 Free PMC article.
-
PLGA-collagen-ECM hybrid scaffolds functionalized with biomimetic extracellular matrices secreted by mesenchymal stem cells during stepwise osteogenesis-co-adipogenesis.J Mater Chem B. 2019 Dec 7;7(45):7195-7206. doi: 10.1039/c9tb01959f. Epub 2019 Oct 29. J Mater Chem B. 2019. PMID: 31660577
-
The role of microRNAs in cell fate determination of mesenchymal stem cells: balancing adipogenesis and osteogenesis.BMB Rep. 2015 Jun;48(6):319-23. doi: 10.5483/bmbrep.2015.48.6.206. BMB Rep. 2015. PMID: 25341923 Free PMC article. Review.
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
Cited by
-
Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs.Stem Cell Res Ther. 2019 Jun 13;10(1):170. doi: 10.1186/s13287-019-1278-x. Stem Cell Res Ther. 2019. PMID: 31196201 Free PMC article.
-
Human cardiomyocyte-derived exosomes induce cardiac gene expressions in mesenchymal stromal cells within 3D hyaluronic acid hydrogels and in dose-dependent manner.J Mater Sci Mater Med. 2021 Jan 19;32(1):2. doi: 10.1007/s10856-020-06474-7. J Mater Sci Mater Med. 2021. PMID: 33469781 Free PMC article.
-
Exosomes Derived from Dental Pulp Stem Cells Show Different Angiogenic and Osteogenic Properties in Relation to the Age of the Donor.Pharmaceutics. 2022 Apr 21;14(5):908. doi: 10.3390/pharmaceutics14050908. Pharmaceutics. 2022. PMID: 35631496 Free PMC article.
-
Natural medicine delivery from 3D printed bone substitutes.J Control Release. 2024 Jan;365:848-875. doi: 10.1016/j.jconrel.2023.09.025. Epub 2023 Dec 17. J Control Release. 2024. PMID: 37734674 Free PMC article. Review.
-
Exosomes as a Novel Approach to Reverse Osteoporosis: A Review of the Literature.Front Bioeng Biotechnol. 2020 Oct 23;8:594247. doi: 10.3389/fbioe.2020.594247. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195163 Free PMC article. Review.
References
-
- Caplan AI. Mesenchymal stem cells. Journal of orthopaedic research 1991;9(5):641–50. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. science 1999;284(5411):143–7. - PubMed
-
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell 2004;6(4):483–95. - PubMed
-
- Narayanan K, Leck K-J, Gao S, Wan AC. Three-dimensional reconstituted extracellular matrix scaffolds for tissue engineering. Biomaterials 2009;30(26):4309–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

