Double-Blind Randomized Clinical Trial of Prazosin for Alcohol Use Disorder
- PMID: 30153753
- PMCID: PMC6395537
- DOI: 10.1176/appi.ajp.2018.17080913
Double-Blind Randomized Clinical Trial of Prazosin for Alcohol Use Disorder
Abstract
Objective: Current medications for alcohol use disorder do not target brain noradrenergic pathways. Theoretical and preclinical evidence suggests that noradrenergic circuits may be involved in alcohol reinforcement and relapse. After a positive pilot study, the authors tested the α-1 adrenergic receptor antagonist prazosin to treat alcohol use disorder in a larger sample.
Method: Ninety-two participants with alcohol use disorder but without posttraumatic stress disorder were randomly assigned to receive prazosin or placebo in a 12-week double-blind study. Medication was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of week 2. The behavioral platform was medical management. Participants provided daily data on alcohol consumption. Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Results: Eighty participants completed the titration period and were included in the primary analyses. There was a significant interaction between condition and week for both number of drinks and number of heavy drinking days, such that the rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants in the prazosin condition compared with those in the placebo condition. Participants in the prazosin condition were more likely to report drowsiness and edema than participants in the placebo condition.
Conclusions: Prazosin holds promise as a harm-reduction pharmacologic treatment for alcohol use disorder and deserves further evaluation by independent research groups.
Trial registration: ClinicalTrials.gov NCT00762710.
Keywords: Alcohol Abuse; Clinical Drug Studies; Noradrenergic; Prazosin.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
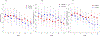
Comment in
-
Prazosin for the Treatment of Alcohol Use Disorders.Am J Psychiatry. 2018 Dec 1;175(12):1159-1160. doi: 10.1176/appi.ajp.2018.18091022. Am J Psychiatry. 2018. PMID: 30501414 No abstract available.
-
Prazosin for Alcohol Use Disorder: Response to Kleinman and Ostacher.Am J Psychiatry. 2019 Feb 1;176(2):165-166. doi: 10.1176/appi.ajp.2018.18101143r. Am J Psychiatry. 2019. PMID: 30704279 No abstract available.
-
Prazosin and Alcohol Use Disorder.Am J Psychiatry. 2019 Feb 1;176(2):165. doi: 10.1176/appi.ajp.2018.18101143. Am J Psychiatry. 2019. PMID: 30704280 No abstract available.
References
-
- Menkes DB, Baraban JM, Aghajanian GK. Prazosin selectively antagonizes neuronal responses mediated by alpha1-adrenoceptors in brain. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:273–275. - PubMed
-
- Funk D, Coen K, Tamadon S, Li Z, Loughlin A, Le AD. Effects of prazosin and doxazosin on yohimbine-induced reinstatement of alcohol seeking in rats. Psychopharmacology (Berl). 2016;233:2197–2207. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

