Intervertebral disc degeneration induced by long-segment in-situ immobilization: a macro, micro, and nanoscale analysis
- PMID: 30153821
- PMCID: PMC6114269
- DOI: 10.1186/s12891-018-2235-z
Intervertebral disc degeneration induced by long-segment in-situ immobilization: a macro, micro, and nanoscale analysis
Abstract
Background: Cervical spine fixation or immobilization has become a routine treatment for spinal fracture, dislocation, subluxation injuries, or spondylosis. The effects of immobilization of intervertebral discs of the cervical spine is unclear. The goal of this study was to evaluate the effects of long-segment in-situ immobilization of intervertebral discs of the caudal vertebra, thereby simulating human cervical spine immobilization.
Methods: Thirty-five fully grown, male Sprague-Dawley rats were used. Rats were randomly assigned to one of five groups: Group A, which served as controls, and Groups B, C, D, and E, in which the caudal vertebrae were in-situ immobilized using a custom-made external device that fixed four caudal vertebrae (Co7-Co10). After 2 weeks, 4 weeks, 6 weeks, and 8 weeks of in-situ immobilization, the caudal vertebrae were harvested, and the disc height, the T2 signal intensity of the discs, disc morphology, the gene expression of discs, and the structure and the elastic modulus of discs was measured.
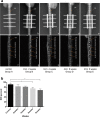
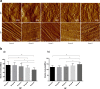
Results: The intervertebral disc height progressively decreased, starting at the 6th week. At week 6 and week 8, disc degeneration was classified as grade III, according to the modified Pfirrmann grading system criteria. Long-segment immobilization altered the gene expression of discs. The nucleus pulposus showed a typical cell cluster phenomenon over time. The annulus fibrosus inner layer began to appear disordered with fissure formation. The elastic modulus of collagen fibrils within the nucleus pulposus was significantly decreased in rats in group E compared to rats in group A (p < 0.05). On the contrary, the elastic modulus within the annulus was significantly increased in rats in group E compared to rats in group A (p < 0.05).
Conclusion: Long-segment in-situ immobilization caused target disc degeneration, and positively correlated with fixation time. The degeneration was not only associated with changes at the macroscale and microscale, but also indicated changes in collagen fibrils at the nanoscale. Long-segment immobilization of the spine (cervical spine) does not seem to be an innocuous strategy for the treatment of spine-related diseases and may be a predisposing factor in the development of the symptomatic spine.
Keywords: Biomechanics; Cervical spine; Fixation; Immobilization; Intervertebral disc degeneration; Rat model.
Conflict of interest statement
Ethics approval and consent to participate
All animal experiments were strictly performed under the guidelines of the Chinese Council for Animal Care, approved by the Animal Care Committee of the Laboratory Animal at School of Medicine, SooChow University(ECSU-201700035).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Stable mechanical environments created by a low-tension traction device is beneficial for the regeneration and repair of degenerated intervertebral discs.Spine J. 2020 Sep;20(9):1503-1516. doi: 10.1016/j.spinee.2020.04.005. Epub 2020 Apr 17. Spine J. 2020. PMID: 32305426
-
Assessment of changes in the micro-nano environment of intervertebral disc degeneration based on Pfirrmann grade.Spine J. 2019 Jul;19(7):1242-1253. doi: 10.1016/j.spinee.2019.01.008. Epub 2019 Jan 30. Spine J. 2019. PMID: 30710732
-
Controlled immobilization-traction based on intervertebral stability is conducive to the regeneration or repair of the degenerative disc: an in vivo study on the rat coccygeal model.Spine J. 2019 May;19(5):920-930. doi: 10.1016/j.spinee.2018.10.018. Epub 2018 Nov 3. Spine J. 2019. PMID: 30399448
-
Intervertebral disc degeneration.Eur Spine J. 1993 Mar;1(4):205-13. doi: 10.1007/BF00298361. Eur Spine J. 1993. PMID: 20054919 Review.
-
A Novel Non-invasive Effective Method for Potential Treatment of Degenerative Disc Disease: A Hypothesis.Cent Nerv Syst Agents Med Chem. 2019;19(1):8-14. doi: 10.2174/1871524918666181017152053. Cent Nerv Syst Agents Med Chem. 2019. PMID: 30332977 Review.
Cited by
-
Regenerating and repairing degenerative intervertebral discs by regulating the micro/nano environment of degenerative bony endplates based on low-tension mechanics.BMC Musculoskelet Disord. 2022 May 16;23(1):462. doi: 10.1186/s12891-022-05422-6. BMC Musculoskelet Disord. 2022. PMID: 35578221 Free PMC article.
-
Insights into the mechanical microenvironment within the cartilaginous endplate: An emerging role in maintaining disc homeostasis and normal function.Heliyon. 2024 May 11;10(10):e31162. doi: 10.1016/j.heliyon.2024.e31162. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803964 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

