Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death
- PMID: 30154112
- PMCID: PMC6238188
- DOI: 10.1182/blood-2018-04-842260
Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death
Abstract
Protein crystallization in human tissue rarely occurs. Charcot-Leyden crystals (CLCs) were described in various eosinophilic diseases >150 years ago, but our understanding of CLC formation still remains limited. In this study, we demonstrate that CLCs observed in varied inflamed human tissues are closely associated with eosinophil cell-free granules and nuclear envelope/plasma membrane disintegration with release of filamentous chromatin (extracellular traps), typical morphologies of a regulated pathway of extracellular trap cell death (ETosis). During the process of eosinophil ETosis, eccentrically localized cytoplasmic and perinuclear CLC protein (galectin-10) is homogeneously redistributed in the cytoplasm. Rapid (1-2 minutes) formation of intracytoplasmic CLCs was observed using time-lapse imaging. Plasma membrane rupture enabled the release of both intracellularly formed CLCs and soluble galectin-10 that further contributed to formation of CLCs extracellularly, in parallel with the expulsion of free intact granules and extracellular traps. CLC formation and galectin-10 release were dependent on nicotinamide adenine dinucleotide phosphate oxidase activation. To our knowledge, this is the first demonstration of natural formation of CLCs in association with an active physiological process (ie, ETosis). These results indicate that dynamic changes in intracellular localization and release of galectin-10 contribute to CLC formation in vivo and suggest that CLC/galectin-10 might serve as an indicator of ETosis.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

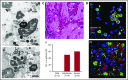
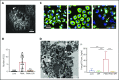
Comment in
-
Charcot-Leyden crystals: solving an enigma.Blood. 2018 Nov 15;132(20):2111-2112. doi: 10.1182/blood-2018-09-873653. Blood. 2018. PMID: 30442747 Free PMC article.
References
-
- Doye J, Poon W. Protein crystallization in vivo. Curr Opin Colloid Interface Sci. 2006;11(1):40-46.
-
- Guo L, Johnson RS, Schuh JC. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J Biol Chem. 2000;275(11):8032-8037. - PubMed
-
- Ayres WW, Starkey NM. Studies on Charcot-Leyden crystals. Blood. 1950;5(3):254-266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

