Subplasmalemmal hydrogen peroxide triggers calcium influx in gonadotropes
- PMID: 30154243
- PMCID: PMC6187636
- DOI: 10.1074/jbc.RA118.001830
Subplasmalemmal hydrogen peroxide triggers calcium influx in gonadotropes
Abstract
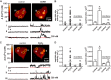
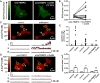
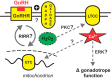
Gonadotropin-releasing hormone (GnRH) stimulation of its eponymous receptor on the surface of endocrine anterior pituitary gonadotrope cells (gonadotropes) initiates multiple signaling cascades that culminate in the secretion of luteinizing and follicle-stimulating hormones, which have critical roles in fertility and reproduction. Enhanced luteinizing hormone biosynthesis, a necessary event for ovulation, requires a signaling pathway characterized by calcium influx through L-type calcium channels and subsequent activation of the mitogen-activated protein kinase extracellular signal-regulated kinase (ERK). We previously reported that highly localized subplasmalemmal calcium microdomains produced by L-type calcium channels (calcium sparklets) play an essential part in GnRH-dependent ERK activation. Similar to calcium, reactive oxygen species (ROS) are ubiquitous intracellular signaling molecules whose subcellular localization determines their specificity. To investigate the potential influence of oxidant signaling in gonadotropes, here we examined the impact of ROS generation on L-type calcium channel function. Total internal reflection fluorescence (TIRF) microscopy revealed that GnRH induces spatially restricted sites of ROS generation in gonadotrope-derived αT3-1 cells. Furthermore, GnRH-dependent stimulation of L-type calcium channels required intracellular hydrogen peroxide signaling in these cells and in primary mouse gonadotropes. NADPH oxidase and mitochondrial ROS generation were each necessary for GnRH-mediated stimulation of L-type calcium channels. Congruently, GnRH increased oxidation within subplasmalemmal mitochondria, and L-type calcium channel activity correlated strongly with the presence of adjacent mitochondria. Collectively, our results provide compelling evidence that NADPH oxidase activity and mitochondria-derived hydrogen peroxide signaling play a fundamental role in GnRH-dependent stimulation of L-type calcium channels in anterior pituitary gonadotropes.
Keywords: NADPH oxidase; calcium imaging; cell signaling; mitochondria; reactive oxygen species (ROS).
© 2018 Dang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

