Co-Amorphous Simvastatin-Nifedipine with Enhanced Solubility for Possible Use in Combination Therapy of Hypertension and Hypercholesterolemia
- PMID: 30154310
- PMCID: PMC6225140
- DOI: 10.3390/molecules23092161
Co-Amorphous Simvastatin-Nifedipine with Enhanced Solubility for Possible Use in Combination Therapy of Hypertension and Hypercholesterolemia
Abstract
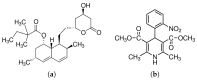
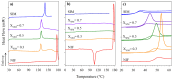
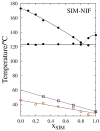
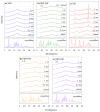
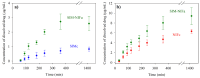
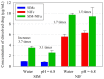
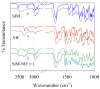
The high index of simultaneous incidence of hypertension and hypercholesterolemia in the population of many countries demands the preparation of more efficient drugs. Therefore, there is a significant area of opportunity to provide as many alternatives as possible to treat these illnesses. Taking advantage of the solubility enhancement that can be achieved when an active pharmaceutical ingredient (API) is obtained and stabilized in its amorphous state, in the present work, new drug-drug co-amorphous formulations (Simvastatin SIM- Nifedipine NIF) with enhanced solubility and stability were prepared and characterized. Results show that the co-amorphous system (molar ratio 1:1) is more soluble than the pure commercial APIs studied separately. Aqueous dissolution profiles showed increments of solubility of 3.7 and 1.7 times for SIM and NIF, correspondingly, in the co-amorphous system. The new co-amorphous formulations, monitored in time, (molar fractions 0.3, 0.5 and 0.7 of SIM) remained stable in the amorphous state for more than one year when stored at room temperature and did not show any signs of crystallization when re-heating. Inspection on the remainder of a sample after six hours of dissolution showed no recrystallization, confirming the stability of co-amorphous system. The enhanced solubility of the co-amorphous formulations makes them promising for simultaneously targeting of hypertension and hypercholesterolemia through combination therapy.
Keywords: Nifedipine; Simvastatin; amorphous drug; co-amorphous; hypercholesterolemia; hypertension; solubility.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

References
-
- Ramirez E., Laosa O., Guerra P., Duque B., Mosquera B., Borobia A.M., Lei S.H., Carcas A.J., Frias J. Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the Biopharmaceutic Classification System. Br. J. Clin. Pharmacol. 2010;70:694–702. doi: 10.1111/j.1365-2125.2010.03757.x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

