Centrosomal and Non-Centrosomal Microtubule-Organizing Centers (MTOCs) in Drosophila melanogaster
- PMID: 30154378
- PMCID: PMC6162459
- DOI: 10.3390/cells7090121
Centrosomal and Non-Centrosomal Microtubule-Organizing Centers (MTOCs) in Drosophila melanogaster
Abstract
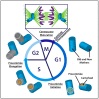
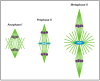
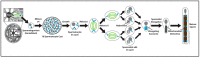
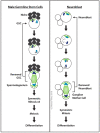
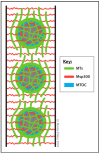
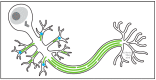
The centrosome is the best-understood microtubule-organizing center (MTOC) and is essential in particular cell types and at specific stages during Drosophila development. The centrosome is not required zygotically for mitosis or to achieve full animal development. Nevertheless, centrosomes are essential maternally during cleavage cycles in the early embryo, for male meiotic divisions, for efficient division of epithelial cells in the imaginal wing disc, and for cilium/flagellum assembly in sensory neurons and spermatozoa. Importantly, asymmetric and polarized division of stem cells is regulated by centrosomes and by the asymmetric regulation of their microtubule (MT) assembly activity. More recently, the components and functions of a variety of non-centrosomal microtubule-organizing centers (ncMTOCs) have begun to be elucidated. Throughout Drosophila development, a wide variety of unique ncMTOCs form in epithelial and non-epithelial cell types at an assortment of subcellular locations. Some of these cell types also utilize the centrosomal MTOC, while others rely exclusively on ncMTOCs. The impressive variety of ncMTOCs being discovered provides novel insight into the diverse functions of MTOCs in cells and tissues. This review highlights our current knowledge of the composition, assembly, and functional roles of centrosomal and non-centrosomal MTOCs in Drosophila.
Keywords: Drosophila; centriole; centrosome; microtubule; microtubule-organizing center (MTOC); ninein; non-centrosomal MTOC; patronin; γ-tubulin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cooper G.M., Hausman R.E. The Cell: A Molecular Approach. 6th ed. Sinauer Associates, Inc.; Sunderland, MA, USA: 2013.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

