Corneal myofibroblasts inhibit regenerating nerves during wound healing
- PMID: 30154512
- PMCID: PMC6113331
- DOI: 10.1038/s41598-018-30964-y
Corneal myofibroblasts inhibit regenerating nerves during wound healing
Abstract
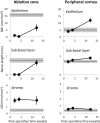
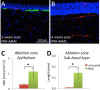
Abnormal nerve regeneration often follows corneal injury, predisposing patients to pain, dry eye and vision loss. Yet, we lack a mechanistic understanding of this process. A key event in corneal wounds is the differentiation of keratocytes into fibroblasts and scar-forming myofibroblasts. Here, we show for the first time that regenerating nerves avoid corneal regions populated by myofibroblasts in vivo. Recreating this interaction in vitro, we find neurite outgrowth delayed when myofibroblasts but not fibroblasts, are co-cultured with sensory neurons. After neurites elongated sufficiently, contact inhibition was observed with myofibroblasts, but not fibroblasts. Reduced neurite outgrowth in vitro appeared mediated by transforming growth factor beta 1 (TGF-β1) secreted by myofibroblasts, which increased phosphorylation of collapsin response mediating protein 2 (CRMP2) in neurons. The significance of this mechanism was further tested by applying Mitomycin C after photorefractive keratectomy to decrease myofibroblast differentiation. This generated earlier repopulation of the ablation zone by intra-epithelial and sub-basal nerves. Our findings suggest that attaining proper, rapid corneal nerve regeneration after injury may require blocking myofibroblast differentiation and/or TGF-β during wound healing. They also highlight hitherto undefined myofibroblast-neuron signaling processes capable of restricting neurite outgrowth in the cornea and other tissues where scars and nerves co-exist.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

