Multifunctional Nanotherapeutics for the Treatment of neuroAIDS in Drug Abusers
- PMID: 30154522
- PMCID: PMC6113246
- DOI: 10.1038/s41598-018-31285-w
Multifunctional Nanotherapeutics for the Treatment of neuroAIDS in Drug Abusers
Abstract
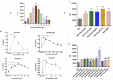
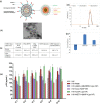
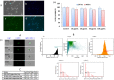
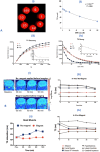
HIV and substance abuse plays an important role in infection and disease progression. Further, the presence of persistent viral CNS reservoirs makes the complete eradication difficult. Thus, neutralizing the drug of abuse effect on HIV-1 infectivity and elimination of latently infected cells is a priority. The development of a multi-component [antiretroviral drugs (ARV), latency reactivating agents (LRA) and drug abuse antagonist (AT)] sustained release nanoformulation targeting the CNS can overcome the issues of HIV-1 cure and will help in improving the drug adherence. The novel magneto-liposomal nanoformulation (NF) was developed to load different types of drugs (LRAs, ARVs, and Meth AT) and evaluated for in-vitro and in-vivo BBB transmigration and antiviral efficacy in primary CNS cells. We established the HIV-1 latency model using human astrocyte cells (HA) and optimized the dose of LRA for latency reversal, Meth AT in in-vitro cell culture system. Further, PEGylated magneto-liposomal NF was developed, characterized for size, shape, drug loading and BBB transport in-vitro. Results showed that drug released in a sustained manner up to 10 days and able to reduce the HIV-1 infectivity up to ~40-50% (>200 pg/mL to <100 pg/mL) continuously using single NF treatment ± Meth treatment in-vitro. The magnetic treatment (0.8 T) was able to transport (15.8% ± 5.5%) NF effectively without inducing any toxic effects due to NF presence in the brain. Thus, our approach and result showed a way to eradicate HIV-1 reservoirs from the CNS and possibility to improve the therapeutic adherence to drugs in drug abusing (Meth) population. In conclusion, the developed NF can provide a better approach for the HIV-1 cure and a foundation for future HIV-1 purging strategies from the CNS using nanotechnology platform.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Nath A, Sacktor N. Influence of highly active antiretroviral therapy on persistence of HIV in the central nervous system. Current opinion in neurology. 2006;19:358–361. doi: 10.1097/01.wco.0000236614.51592.ca. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA042706/DA/NIDA NIH HHS/United States
- R01DA034547/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
- R01DA027049/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
- R01DA037838/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
- R01 DA037838/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

