Use of sucrose to diminish pore formation in freeze-dried heart valves
- PMID: 30154529
- PMCID: PMC6113295
- DOI: 10.1038/s41598-018-31388-4
Use of sucrose to diminish pore formation in freeze-dried heart valves
Abstract
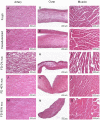
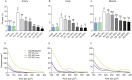
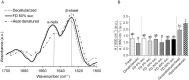
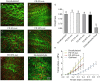
Freeze-dried storage of decellularized heart valves provides easy storage and transport for clinical use. Freeze-drying without protectants, however, results in a disrupted histoarchitecture after rehydration. In this study, heart valves were incubated in solutions of various sucrose concentrations and subsequently freeze-dried. Porosity of rehydrated valves was determined from histological images. In the absence of sucrose, freeze-dried valves were shown to have pores after rehydration in the cusp, artery and muscle sections. Use of sucrose reduced pore formation in a dose-dependent manner, and pretreatment of the valves in a 40% (w/v) sucrose solution prior to freeze-drying was found to be sufficient to completely diminish pore formation. The presence of pores in freeze-dried valves was found to coincide with altered biomechanical characteristics, whereas biomechanical parameters of valves freeze-dried with enough sucrose were not significantly different from those of valves not exposed to freeze-drying. Multiphoton imaging, Fourier transform infrared spectroscopy, and differential scanning calorimetry studies revealed that matrix proteins (i.e. collagen and elastin) were not affected by freeze-drying.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hammermeister, K. et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: Final report of the veterans affairs randomized trial. J. Am. Coll. Cardiol.36, 1152–1158 (2000). - PubMed
-
- Nachlas, A. L. Y., Li, S. & Davis, M. E. Developing a clinically relevant tissue engineered heart valve-A review of current approaches. Adv. Healthc. Mater.6, 1700918 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

