Decrease in plasma membrane tension triggers PtdIns(4,5)P2 phase separation to inactivate TORC2
- PMID: 30154550
- PMCID: PMC6237274
- DOI: 10.1038/s41556-018-0150-z
Decrease in plasma membrane tension triggers PtdIns(4,5)P2 phase separation to inactivate TORC2
Abstract
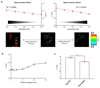
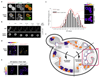
The target of rapamycin complex 2 (TORC2) plays a key role in maintaining the homeostasis of plasma membrane (PM) tension. TORC2 activation following increased PM tension involves redistribution of the Slm1 and 2 paralogues from PM invaginations known as eisosomes into membrane compartments containing TORC2. How Slm1/2 relocalization is triggered, and if/how this plays a role in TORC2 inactivation with decreased PM tension, is unknown. Using osmotic shocks and palmitoylcarnitine as orthogonal tools to manipulate PM tension, we demonstrate that decreased PM tension triggers spontaneous, energy-independent reorganization of pre-existing phosphatidylinositol-4,5-bisphosphate into discrete invaginated membrane domains, which cluster and inactivate TORC2. These results demonstrate that increased and decreased membrane tension are sensed through different mechanisms, highlighting a role for membrane lipid phase separation in mechanotransduction.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2.Mol Biol Cell. 2005 Apr;16(4):1883-900. doi: 10.1091/mbc.e04-07-0564. Epub 2005 Feb 2. Mol Biol Cell. 2005. PMID: 15689497 Free PMC article.
-
Analysis of the roles of phosphatidylinositol-4,5-bisphosphate and individual subunits in assembly, localization, and function of Saccharomyces cerevisiae target of rapamycin complex 2.Mol Biol Cell. 2019 Jun 1;30(12):1555-1574. doi: 10.1091/mbc.E18-10-0682. Epub 2019 Apr 10. Mol Biol Cell. 2019. PMID: 30969890 Free PMC article.
-
Eisosomes at the intersection of TORC1 and TORC2 regulation.Traffic. 2019 Aug;20(8):543-551. doi: 10.1111/tra.12651. Epub 2019 May 30. Traffic. 2019. PMID: 31038844 Free PMC article. Review.
-
Plasma membrane proteins Slm1 and Slm2 mediate activation of the AGC kinase Ypk1 by TORC2 and sphingolipids in S. cerevisiae.Cell Cycle. 2012 Oct 15;11(20):3745-9. doi: 10.4161/cc.21752. Epub 2012 Aug 16. Cell Cycle. 2012. PMID: 22895050 Free PMC article.
-
Regulation of TORC2 function and localization by Rab5 GTPases in Saccharomyces cerevisiae.Cell Cycle. 2019 May;18(10):1084-1094. doi: 10.1080/15384101.2019.1616999. Epub 2019 May 15. Cell Cycle. 2019. PMID: 31068077 Free PMC article. Review.
Cited by
-
An Excitable Ras/PI3K/ERK Signaling Network Controls Migration and Oncogenic Transformation in Epithelial Cells.Dev Cell. 2020 Sep 14;54(5):608-623.e5. doi: 10.1016/j.devcel.2020.08.001. Epub 2020 Sep 1. Dev Cell. 2020. PMID: 32877650 Free PMC article.
-
Genome-Wide Analysis of AGC Kinases Reveals that MoFpk1 Is Required for Development, Lipid Metabolism, and Autophagy in Hyperosmotic Stress of the Rice Blast Fungus Magnaporthe oryzae.mBio. 2022 Dec 20;13(6):e0227922. doi: 10.1128/mbio.02279-22. Epub 2022 Oct 19. mBio. 2022. PMID: 36259725 Free PMC article.
-
Piezo1 activation attenuates thrombin-induced blebbing in breast cancer cells.J Cell Sci. 2022 Apr 1;135(7):jcs258809. doi: 10.1242/jcs.258809. Epub 2022 Apr 1. J Cell Sci. 2022. PMID: 35274124 Free PMC article.
-
Yeast osmoregulation - glycerol still in pole position.FEMS Yeast Res. 2022 Aug 30;22(1):foac035. doi: 10.1093/femsyr/foac035. FEMS Yeast Res. 2022. PMID: 35927716 Free PMC article. Review.
-
Notch ankyrin domain: evolutionary rise of a thermodynamic sensor.Cell Commun Signal. 2022 May 18;20(1):66. doi: 10.1186/s12964-022-00886-4. Cell Commun Signal. 2022. PMID: 35585601 Free PMC article.
References
Methods only References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

