2-Methoxyestradiol Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats through Inhibition of HIF-1 α/TGF- β/Smad2 Axis
- PMID: 30154949
- PMCID: PMC6093036
- DOI: 10.1155/2018/4389484
2-Methoxyestradiol Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats through Inhibition of HIF-1 α/TGF- β/Smad2 Axis
Abstract
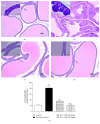
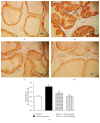
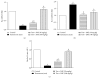
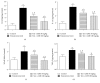
Benign prostatic hyperplasia (BPH) is a common disorder in the male population. 2-Methoxyestradiol (2ME) is an end metabolite of estrogens with pleiotropic pharmacological properties. This study aimed to explore the potential ameliorative effects of 2ME against testosterone-induced BPH in rats. 2-Methoxyestradiol (50 and 100 mg/kg, dissolved in DMSO) prevented the rise in prostatic index and weight in comparison to testosterone-alone-treated animals for 2 weeks. Histological examination indicated that 2ME ameliorated pathological changes in prostate architecture. This was confirmed by the ability of 2ME to decrease the glandular epithelial height when compared to the testosterone group. Also, 2ME improved testosterone-induced oxidative stress as it inhibited the rise in lipid peroxide content and the exhaustion of superoxide dismutase (SOD) activity. The beneficial effects of 2ME against the development of BPH were substantiated by assessing proliferation markers, preventing the rise in cyclin D1 protein expression and enhancing Bax/Bcl2 mRNA ratio. It significantly reduced prostate content of tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), nuclear factor κB (NF-κB), and transforming growth factor β (TGF-β). In addition, 2ME reduced hypoxia-inducible factor 1-α (HIF-1α) and phospho-Smad2 (p-Smad2) protein expression compared to the testosterone group. In conclusion, 2ME attenuates experimentally induced BPH by testosterone in rats through, at least partly, inhibition of HIF-1α/TGF-β/Smad2 axis.
Figures

References
-
- Park H. J., Won J. E. J., Sorsaburu S., Rivera P. D., Lee S. W. Urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) and LUTS/BPH with erectile dysfunction in Asian men: a systematic review focusing on tadalafil. The World Journal of Men's Health. 2013;31(3):193–207. doi: 10.5534/wjmh.2013.31.3.193. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

