Rapid biocompatible macrocyclization of peptides with decafluoro-diphenylsulfone
- PMID: 30155020
- PMCID: PMC6013815
- DOI: 10.1039/c5sc03856a
Rapid biocompatible macrocyclization of peptides with decafluoro-diphenylsulfone
Erratum in
-
Correction: Rapid biocompatible macrocyclization of peptides with decafluoro-diphenylsulfone.Chem Sci. 2017 Jan 1;8(1):807. doi: 10.1039/c6sc90071b. Epub 2016 Nov 11. Chem Sci. 2017. PMID: 30124676 Free PMC article.
Abstract
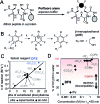
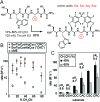
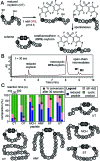
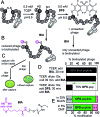
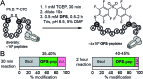
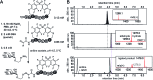
In this manuscript, we describe modification of Cys-residues in peptides and proteins in aqueous solvents via aromatic nucleophilic substitution (SNAr) with perfluoroarenes (fAr). Biocompatibility of this reaction makes it attractive for derivatization of proteins and peptide libraries comprised of 20 natural amino acids. Measurement of the reaction rates for fAr derivatives by 19F NMR with a model thiol donor (β-mercaptoethanol) in aqueous buffers identified decafluoro-diphenylsulfone (DFS) as the most reactive SNAr electrophile. Reaction of DFS with thiol nucleophiles is >100 000 faster than analogous reaction of perfluorobenzene; this increase in reactivity enables application of DFS at low concentrations in aqueous solutions compatible with biomolecules and protein complexes irreversibly degraded by organic solvents (e.g., bacteriophages). DFS forms macrocycles when reacted with peptides of the general structure X n -Cys-X m -Cys-X l , where X is any amino acid and m = 1-15. It formed cyclic peptides with 6 peptide hormones-oxytocin, urotensin II, salmon calcitonin, melanin-concentrating hormone, somatostatin-14, and atrial natriuretic factor (1-28) as well as peptides displayed on M13 phage. Rates up to 180 M-1 s-1 make this reaction one of the fastest Cys-modifications to-date. Long-term stability of macrocycles derived from DFS and their stability toward oxidation further supports DFS as a promising method for modification of peptide-based ligands, cyclization of genetically-encoded peptide libraries, and discovery of bioactive macrocyclic peptides.
Figures
References
-
- Bhat A., Roberts L. R., Dwyer J. J. Eur. J. Med. Chem. 2015;94:471–479. - PubMed
-
- Hackenberger C. P. R., Schwarzer D. Angew. Chem., Int. Ed. 2008;47:10030–10074. - PubMed
-
- Tsomaia N. Eur. J. Med. Chem. 2015;94:459–470. - PubMed
-
- White C. J., Yudin A. K. Nat. Chem. 2011;3:509–524. - PubMed
-
- Azzarito V., Long K., Murphy N. S., Wilson A. J. Nat. Chem. 2013;5:161–173. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

