Macrocyclic squaramides: anion receptors with high sulfate binding affinity and selectivity in aqueous media
- PMID: 30155103
- PMCID: PMC6014085
- DOI: 10.1039/c6sc01011c
Macrocyclic squaramides: anion receptors with high sulfate binding affinity and selectivity in aqueous media
Abstract
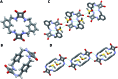
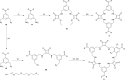
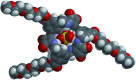
A number of macrocyclic squaramide-containing receptors (MSQs) have been designed and synthesised and their interaction with a range of inorganic anions was studied in solution by 1H NMR spectroscopy and ESI-HRMS. The binding data revealed remarkable binding of sulfate in aqueous mixtures from 0.5 to 50% v/v H2O/DMSO-d6. The larger [3]-MSQs were found to better match the size and shape of the sulfate ion than the [2]-MSQs, providing high affinity and selectivity for sulfate while other tetrahedral divalent anions such as selenate, phosphate species and chromate have substantially lower binding affinities. In mixtures of anions mimicking the composition of either nuclear waste or plasma, the [3]-MSQs were still able to bind sulfate ions with high affinity.
Figures







Similar articles
-
Macrocyclic squaramides as ion pair receptors and fluorescent sensors selective towards sulfates.Dalton Trans. 2021 Mar 21;50(11):3904-3915. doi: 10.1039/d0dt04273k. Epub 2021 Feb 26. Dalton Trans. 2021. PMID: 33635308
-
Sulfate-selective recognition by using neutral dipeptide anion receptors in aqueous solution.Chemistry. 2014 Jun 10;20(24):7373-80. doi: 10.1002/chem.201400292. Epub 2014 May 14. Chemistry. 2014. PMID: 24828677
-
Receptors for sulfate that function across a wide pH range in mixed aqueous-DMSO media.Chem Commun (Camb). 2019 Oct 10;55(82):12312-12315. doi: 10.1039/c9cc06812k. Chem Commun (Camb). 2019. PMID: 31559993
-
Anion Recognition in Aqueous Media by Cyclopeptides and Other Synthetic Receptors.Acc Chem Res. 2017 Nov 21;50(11):2870-2878. doi: 10.1021/acs.accounts.7b00458. Epub 2017 Nov 10. Acc Chem Res. 2017. PMID: 29125287 Review.
-
Bacterial transport of sulfate, molybdate, and related oxyanions.Biometals. 2011 Aug;24(4):687-707. doi: 10.1007/s10534-011-9421-x. Epub 2011 Feb 8. Biometals. 2011. PMID: 21301930 Review.
Cited by
-
Squaramide-Based Self-Associating Amphiphiles for Anion Recognition.Chempluschem. 2021 Aug;86(8):1058-1068. doi: 10.1002/cplu.202100275. Chempluschem. 2021. PMID: 34351081 Free PMC article.
-
Anion-exchange facilitated selective extraction of sulfate and phosphate by overcoming the Hofmeister bias.RSC Adv. 2023 May 31;13(24):16185-16195. doi: 10.1039/d3ra01771k. eCollection 2023 May 30. RSC Adv. 2023. PMID: 37266508 Free PMC article.
-
Uncovering the potent antimicrobial activity of squaramide based anionophores - chloride transport and membrane disruption.Chem Sci. 2025 Jan 22;16(9):4075-4084. doi: 10.1039/d4sc01693a. eCollection 2025 Feb 26. Chem Sci. 2025. PMID: 39906384 Free PMC article.
-
Anion-templated synthesis of a switchable fluorescent [2]catenane with sulfate sensing capability.Chem Sci. 2023 Dec 18;15(5):1796-1809. doi: 10.1039/d3sc05086f. eCollection 2024 Jan 31. Chem Sci. 2023. PMID: 38303949 Free PMC article.
-
Polymeric Materials and Microfabrication Techniques for Liquid Filtration Membranes.Polymers (Basel). 2022 Sep 27;14(19):4059. doi: 10.3390/polym14194059. Polymers (Basel). 2022. PMID: 36236007 Free PMC article. Review.
References
-
- Ravikumar I., Ghosh P. Chem. Soc. Rev. 2012;41:3077–3098. - PubMed
-
- Moyer B. A. and Singh R. P., Fundam. Appl. Anion Sep., Springer, 2004.
-
- Katayev E. A., Ustynyuk Y. A., Sessler J. L. Coord. Chem. Rev. 2006;250:3004–3037.
-
- Olomu A. B., Vickers C. R., Waring R. H., Clements D., Babbs C., Warnes T. W., Elias E. N. Engl. J. Med. 1988;318:1089–1092. - PubMed
-
- Amerongen A. V. N., Bolscher J. G. M., Bloemena E., Veerman E. C. I. Biol. Chem. 1998;379:1–26. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

