Cross-linking and other structural proteomics techniques: how chemistry is enabling mass spectrometry applications in structural biology
- PMID: 30155128
- PMCID: PMC6016523
- DOI: 10.1039/c5sc04196a
Cross-linking and other structural proteomics techniques: how chemistry is enabling mass spectrometry applications in structural biology
Abstract
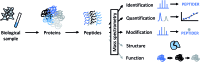
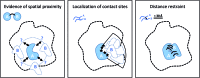
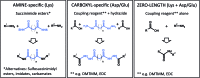
The biological function of proteins is heavily influenced by their structures and their organization into assemblies such as protein complexes and regulatory networks. Mass spectrometry (MS) has been a key enabling technology for high-throughput and comprehensive protein identification and quantification on a proteome-wide scale. Besides these essential contributions, MS can also be used to study higher-order structures of biomacromolecules in a variety of ways. In one approach, intact proteins or protein complexes may be directly probed in the mass spectrometer. Alternatively, various forms of solution-phase chemistry are used to introduce modifications in intact proteins and localizing these modifications by MS analysis at the peptide level is used to derive structural information. Here, I will put a spotlight on the central role of chemistry in such mass spectrometry-based methods that bridge proteomics and structural biology, with a particular emphasis on chemical cross-linking of protein complexes.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

