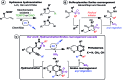
Visible light amination/Smiles cascade: access to phthalazine derivatives
- PMID: 30155150
- PMCID: PMC6018559
- DOI: 10.1039/c6sc01095d
Visible light amination/Smiles cascade: access to phthalazine derivatives
Abstract
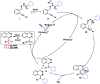
We report the synthesis of various phthalazines via a new cascade reaction, initiated by visible light photocatalysis, involving a radical hydroamination reaction followed by a radical Smiles rearrangement. Phthalazine derivatives are obtained in high yields and from a broad scope readily accessible ortho-alkynylsulfonohydrazone precursors. The mild photoredox conditions ensure an excellent functional group tolerance. Application of this strategy to a one-pot protocol starting from the corresponding carbonyl compounds, and subsequent functionalization allow the rapid synthesis of structurally diverse structures.
Figures
Similar articles
-
Light-Enabled Radical 1,4-Aryl Migration Via a Phospho-Smiles Rearrangement.J Org Chem. 2021 Mar 5;86(5):3758-3767. doi: 10.1021/acs.joc.0c02540. Epub 2021 Jan 13. J Org Chem. 2021. PMID: 33439649
-
Ynamide Smiles Rearrangement Triggered by Visible-Light-Mediated Regioselective Ketyl-Ynamide Coupling: Rapid Access to Functionalized Indoles and Isoquinolines.J Am Chem Soc. 2020 Feb 19;142(7):3636-3644. doi: 10.1021/jacs.9b13975. Epub 2020 Feb 10. J Am Chem Soc. 2020. PMID: 32003986
-
Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis and Functionalization: Reaction Design and Beyond.Acc Chem Res. 2016 Sep 20;49(9):1911-23. doi: 10.1021/acs.accounts.6b00254. Epub 2016 Aug 23. Acc Chem Res. 2016. PMID: 27551740
-
Synthesis of Cyclic Compounds via Photoinduced Radical Cyclization Cascade of C=C bonds.Chem Rec. 2019 Feb;19(2-3):424-439. doi: 10.1002/tcr.201800050. Epub 2018 Jul 18. Chem Rec. 2019. PMID: 30019439 Review.
-
Synthesis and biological activity of structurally diverse phthalazine derivatives: A systematic review.Bioorg Med Chem. 2019 Sep 15;27(18):3979-3997. doi: 10.1016/j.bmc.2019.07.050. Epub 2019 Aug 1. Bioorg Med Chem. 2019. PMID: 31401008
Cited by
-
Triarylmethanes and their Medium-Ring Analogues by Unactivated Truce-Smiles Rearrangement of Benzanilides.Angew Chem Int Ed Engl. 2021 May 10;60(20):11272-11277. doi: 10.1002/anie.202102192. Epub 2021 Apr 8. Angew Chem Int Ed Engl. 2021. PMID: 33830592 Free PMC article.
-
Photocatalyzed cycloaromatization of vinylsilanes with arylsulfonylazides.Nat Commun. 2021 Jun 3;12(1):3304. doi: 10.1038/s41467-021-23326-2. Nat Commun. 2021. PMID: 34083532 Free PMC article.
-
Cerium photocatalyzed radical smiles rearrangement of 2-aryloxybenzoic acids.RSC Adv. 2021 Jul 21;11(41):25207-25210. doi: 10.1039/d1ra04130d. eCollection 2021 Jul 19. RSC Adv. 2021. PMID: 35478894 Free PMC article.
-
Strategies to Generate Nitrogen-centered Radicals That May Rely on Photoredox Catalysis: Development in Reaction Methodology and Applications in Organic Synthesis.Chem Rev. 2022 Jan 26;122(2):2353-2428. doi: 10.1021/acs.chemrev.1c00444. Epub 2021 Oct 8. Chem Rev. 2022. PMID: 34623809 Free PMC article. Review.
-
Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis.Chem Rev. 2022 Jan 26;122(2):2017-2291. doi: 10.1021/acs.chemrev.1c00374. Epub 2021 Nov 23. Chem Rev. 2022. PMID: 34813277 Free PMC article. Review.
References
-
- Heterocycles in Natural Product Synthesis, ed. K. C. Majumdar and S. K. Chattopadhyay, Wiley, Hoboken, NJ, 2012.
- Horton D. A., Bourne G. T., Smythe M. L. Chem. Rev. 2003;103:893. - PubMed
- Deiters A., Martin S. F. Chem. Rev. 2004;104:2199. - PubMed
- de Sá Alves F. R., Barreiro E. J., Fraga C. A. Mini-Rev. Med. Chem. 2009;9:782. - PubMed
- Brancale A., Silvestri R. Med. Res. Rev. 2007;27:209. - PubMed
- Zeni G., Larock R. Chem. Rev. 2004;104:2285. - PubMed
-
- Ibrahim H. S., Eldehna W. M., Abdel-Aziz H. A., Elaasser M. M., Abdel-Aziz M. M. Eur. J. Med. Chem. 2014;85:480. - PubMed
-
- Scott E. N., Meinhardt G., Jacques C., Laurent D., Thomas A. L. Expert Opin. Invest. Drugs. 2007;16:367. - PubMed
-
- Asif M. Curr. Med. Chem. 2012;19:2984. - PubMed
- Dong C., Liao Z., Xu X., Zhou H. J. Heterocycl. Chem. 2014;51:1282.
- Munína J., Santanab L., Uriarteb E., Borgesc F., Quezada E. Tetrahedron Lett. 2015;56:828.
- Maki Y., Furuta T., Suzuki M. J. Chem. Soc., Perkin Trans. 1. 1979:553.
- Heinisch G., Huber T. J. Heterocycl. Chem. 2009;26:1787.
LinkOut - more resources
Full Text Sources
Other Literature Sources