Visible light amination/Smiles cascade: access to phthalazine derivatives
- PMID: 30155150
- PMCID: PMC6018559
- DOI: 10.1039/c6sc01095d
Visible light amination/Smiles cascade: access to phthalazine derivatives
Abstract
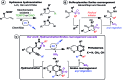
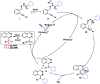
We report the synthesis of various phthalazines via a new cascade reaction, initiated by visible light photocatalysis, involving a radical hydroamination reaction followed by a radical Smiles rearrangement. Phthalazine derivatives are obtained in high yields and from a broad scope readily accessible ortho-alkynylsulfonohydrazone precursors. The mild photoredox conditions ensure an excellent functional group tolerance. Application of this strategy to a one-pot protocol starting from the corresponding carbonyl compounds, and subsequent functionalization allow the rapid synthesis of structurally diverse structures.
Figures
References
-
- Heterocycles in Natural Product Synthesis, ed. K. C. Majumdar and S. K. Chattopadhyay, Wiley, Hoboken, NJ, 2012.
- Horton D. A., Bourne G. T., Smythe M. L. Chem. Rev. 2003;103:893. - PubMed
- Deiters A., Martin S. F. Chem. Rev. 2004;104:2199. - PubMed
- de Sá Alves F. R., Barreiro E. J., Fraga C. A. Mini-Rev. Med. Chem. 2009;9:782. - PubMed
- Brancale A., Silvestri R. Med. Res. Rev. 2007;27:209. - PubMed
- Zeni G., Larock R. Chem. Rev. 2004;104:2285. - PubMed
-
- Ibrahim H. S., Eldehna W. M., Abdel-Aziz H. A., Elaasser M. M., Abdel-Aziz M. M. Eur. J. Med. Chem. 2014;85:480. - PubMed
-
- Scott E. N., Meinhardt G., Jacques C., Laurent D., Thomas A. L. Expert Opin. Invest. Drugs. 2007;16:367. - PubMed
-
- Asif M. Curr. Med. Chem. 2012;19:2984. - PubMed
- Dong C., Liao Z., Xu X., Zhou H. J. Heterocycl. Chem. 2014;51:1282.
- Munína J., Santanab L., Uriarteb E., Borgesc F., Quezada E. Tetrahedron Lett. 2015;56:828.
- Maki Y., Furuta T., Suzuki M. J. Chem. Soc., Perkin Trans. 1. 1979:553.
- Heinisch G., Huber T. J. Heterocycl. Chem. 2009;26:1787.
LinkOut - more resources
Full Text Sources
Other Literature Sources