Total synthesis of aristolactam alkaloids via synergistic C-H bond activation and dehydro-Diels-Alder reactions
- PMID: 30155216
- PMCID: PMC6100235
- DOI: 10.1039/c7sc00161d
Total synthesis of aristolactam alkaloids via synergistic C-H bond activation and dehydro-Diels-Alder reactions
Abstract
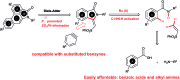
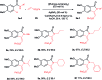
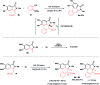
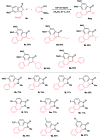
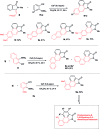
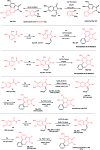
A concise total synthesis of aristolactam alkaloids by a synergistic combination of C-H bond activation and dehydro-Diels-Alder reactions is described. To achieve the synthesis two new synthetic methodologies, namely the oxidative cyclization of benzamides with vinyl sulfone leading to 3-methyleneisoindolin-1-ones via a ruthenium-catalyzed C-H bond activation, and a dehydro-Diels-Alder reaction followed by the fluoride ion mediated desulfonylation of 3-methyleneisoindolin-1-ones with benzynes, were developed. The method presented allows the opportunity for the construction of all the rings of aristolactams from easily available starting materials.
Figures



Similar articles
-
Recent Progress in Dehydro(genative) Diels-Alder Reaction.Chemistry. 2016 Jan 26;22(5):1558-71. doi: 10.1002/chem.201503571. Epub 2015 Nov 24. Chemistry. 2016. PMID: 26786814 Review.
-
Tandem oxidative dearomatization/intramolecular Diels-Alder reaction for construction of the tricyclic core of palhinine A.Org Lett. 2012 May 4;14(9):2293-5. doi: 10.1021/ol3007138. Epub 2012 Apr 12. Org Lett. 2012. PMID: 22497445
-
Recent advancements in the chemistry of Diels-Alder reaction for total synthesis of natural products: a comprehensive review (2020-2023).RSC Adv. 2025 Feb 10;15(6):4496-4525. doi: 10.1039/d4ra07989b. eCollection 2025 Feb 6. RSC Adv. 2025. PMID: 39931410 Free PMC article. Review.
-
Manganese(I)-Catalyzed C-H Activation/Diels-Alder/retro-Diels-Alder Domino Alkyne Annulation featuring Transformable Pyridines.Angew Chem Int Ed Engl. 2019 Apr 8;58(16):5338-5342. doi: 10.1002/anie.201900495. Epub 2019 Mar 18. Angew Chem Int Ed Engl. 2019. PMID: 30753749
-
A 11-Steps Total Synthesis of Magellanine through a Gold(I)-Catalyzed Dehydro Diels-Alder Reaction.Angew Chem Int Ed Engl. 2017 May 22;56(22):6280-6283. doi: 10.1002/anie.201611606. Epub 2017 Jan 12. Angew Chem Int Ed Engl. 2017. PMID: 28079949
Cited by
-
Identification of Aristolactam Derivatives That Act as Inhibitors of Human Immunodeficiency Virus Type 1 Infection and Replication by Targeting Tat-Mediated Viral Transcription.Virol Sin. 2021 Apr;36(2):254-263. doi: 10.1007/s12250-020-00274-7. Epub 2020 Aug 10. Virol Sin. 2021. PMID: 32779073 Free PMC article.
-
Isoindolinone Synthesis via One-Pot Type Transition Metal Catalyzed C-C Bond Forming Reactions.Chemistry. 2021 Mar 22;27(17):5344-5378. doi: 10.1002/chem.202004375. Epub 2021 Feb 2. Chemistry. 2021. PMID: 33125790 Free PMC article. Review.
-
Recent development in transition metal-catalysed C-H olefination.Chem Sci. 2021 Jan 29;12(8):2735-2759. doi: 10.1039/d0sc05555g. Chem Sci. 2021. PMID: 34164039 Free PMC article. Review.
-
Base-Promoted Cascade Reactions for the Synthesis of 3,3-Dialkylated Isoindolin-1-ones and 3-Methyleneisoindolin-1-ones.J Org Chem. 2021 Nov 5;86(21):15128-15138. doi: 10.1021/acs.joc.1c01794. Epub 2021 Oct 6. J Org Chem. 2021. PMID: 34613731 Free PMC article.
References
-
- Choi Y. L., Kim J. K., Choi S.-U., Min Y.-K., Bae M.-A., Kim B. T., Heo J.-N. Bioorg. Med. Chem. Lett. 2009;19:3036. - PubMed
- Desai S. J., Prabhu B. R., Mulchandani N. B. Phytochemistry. 1988;27:1511.
- Zhang Y.-N., Zhong X.-G., Zheng Z.-P., Hu X.-D., Zuo J.-P., Hu L. H. Bioorg. Med. Chem. 2007;15:988. - PubMed
- Houghton P. J., Ogutveren M. Phytochemistry. 1991;30:253. - PubMed
- Vishwanath B. S., Gowda T. V. Toxicon. 1987;25:929. - PubMed
- Priestap H. A. Phytochemistry. 1987;26:519.
-
- Martin F., Choomuenwai V., Davis R. A. Phytochemistry. 2013;86:121. - PubMed
- Kim S. R., Sung S. H., Kang S. Y., Koo K. A., King S. H., Ma C. J., Lee H.-S., Park M. J., Kim Y. C. Planta Med. 2004;70:391. - PubMed
- Likhitwitayawuid K., Wiasathien L., Jongboonprasert V., Krungkrai J., Aimi N., Takayama H., Kitajima M. Pharm. Pharmacol. Lett. 1997;7:99.
- Kim S. K., Ryu S., No Y. J., Choi S. U., Kim Y. S. Arch. Pharmacal Res. 2001;24:518. - PubMed
- Tsai I. L., Lee F. P., Wu C. C., Duh C. Y., Ishikawa T., Chen J. J., Chen Y. C., Seki H., Chen I. S. Planta Med. 2005;71:535. - PubMed
-
- Estevez J. C., Villaverde M. C., Estevez R. J., Castedo L. Tetrahedron. 1995;51:4075.
- Estevez J. C., Estevez R. J., Castedo L. Tetrahedron. 1995;51:10801.
- Gomez B., Guitian E., Castedo L. Synlett. 1992:903.
- Rys V., Couture A., Deniau E., Grandclaudon P. Eur. J. Org. Chem. 2003:1231.
- Hoarau C., Couture A., Cornet H., Deniau E., Grandclaudon P. J. Org. Chem. 2001;66:8064. - PubMed
- Couture A., Deniau E., Grandclaudon P., Hoarau C. J. Org. Chem. 1998;63:3128. - PubMed
- Couture A., Deniau E., Grandclaudon P., Lebrun S. Synlett. 1997:1475.
- Levin J. I., Weinreb S. M. J. Am. Chem. Soc. 1983;105:1397.
- Atanes N., Castedo L., Guitian E., Saa C., Saa J. M., Suau R. J. Org. Chem. 1991;56:2984.
- Kim J. K., Kim Y. H., Nam H. T., Kim B. T., Heo J.-N. Org. Lett. 2008;10:3543. - PubMed
- Heo J.-N., Kim T. J., Kim J. K. Bull. Korean Chem. Soc. 2011;32:4431.
- Sakthivel K., Srinivasan K. Eur. J. Org. Chem. 2013:3386.
- Benesch L., Bury P., Guillaneux D., Houldsworth S., Wang X., Snieckus V. Tetrahedron Lett. 1998;39:961.
- Yao T., Larock R. C. J. Org. Chem. 2005;70:1432. - PubMed
- Estevez J. C., Estevez R. J., Guitihn E., Villaverde M. C., Castedo L. Tetrahedron Lett. 1989;30:5786.
-
-
C–H activation:
- Satoh T., Miura M. Chem.–Eur. J. 2010;16:11212. - PubMed
- Colby D. A., Bermann R. G., Ellman J. A. Chem. Rev. 2010;110:624. - PMC - PubMed
- Zhu C., Wang R., Flack J. R. Chem.–Asian J. 2012;7:1502. - PubMed
- Arokiam P. B., Bruneau C., Dixneuf P. H. Chem. Rev. 2012;112:5879. - PubMed
- Ackermann L. Acc. Chem. Res. 2014;47:281. - PubMed
- Lyons T. W., Sanford M. S. Chem. Rev. 2010;110:1147. - PMC - PubMed
- Engle K. M., Mei T.-S., Wasa M., Yu J.-Q. Acc. Chem. Res. 2012;45:788. - PMC - PubMed
- Bras J. L., Muzart J. Chem. Rev. 2011;111:1170. - PubMed
- Gandeepan P., Cheng C.-H. Chem. Asian. J. 2015;10:824. - PubMed
- Zhu R. Y., Farmer M. E., Chen Y.-Q., Yu J.-Q. Angew. Chem., Int. Ed. 2016;55:10578. - PMC - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

