A landscape view on the interplay between EMT and cancer metastasis
- PMID: 30155271
- PMCID: PMC6107626
- DOI: 10.1038/s41540-018-0068-x
A landscape view on the interplay between EMT and cancer metastasis
Abstract
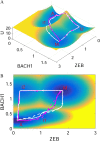
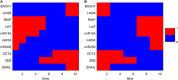
The epithelial-mesenchymal transition (EMT) is a basic developmental process that converts epithelial cells to mesenchymal cells. Although EMT might promote cancer metastasis, the molecular mechanisms for it remain to be fully clarified. To address this issue, we constructed an EMT-metastasis gene regulatory network model and quantified the potential landscape of cancer metastasis-promoting system computationally. We identified four steady-state attractors on the landscape, which separately characterize anti-metastatic (A), metastatic (M), and two other intermediate (I1 and I2) cell states. The tetrastable landscape and the existence of intermediate states are consistent with recent single-cell measurements. We identified one of the two intermediate states I1 as the EMT state. From a MAP approach, we found that for metastatic progression cells need to first undergo EMT (enter the I1 state), and then become metastatic (switch from the I1 state to the M state). Specifically, for metastatic progression, EMT genes (such as ZEB) should be activated before metastasis genes (such as BACH1). This suggests that temporal order is important for the activation of cellular programs in biological systems, and provides a possible mechanism of EMT-promoting cancer metastasis. To identify possible therapeutic targets from this landscape view, we performed sensitivity analysis for individual molecular factors, and identified optimal interventions for landscape control. We found that minimizing transition actions more effectively identifies optimal combinations of targets that induce transitions between attractors than single-factor sensitivity analysis. Overall, the landscape view not only suggests that intermediate states increase plasticity during cell fate decisions, providing a possible source for tumor heterogeneity that is critically important in metastatic progress, but also provides a way to identify therapeutic targets for preventing cancer progression.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

