Evaluation of no evidence of progression or active disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial
- PMID: 30155979
- PMCID: PMC6220799
- DOI: 10.1002/ana.25313
Evaluation of no evidence of progression or active disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial
Abstract
Objective: No evidence of progression or active disease (NEPAD) is a novel combined endpoint defined by the absence of both progression and inflammatory disease activity in primary progressive multiple sclerosis (PPMS). In the placebo-controlled phase III ORATORIO study (NCT01194570), we investigated the effect of ocrelizumab on this comprehensive outcome and its components in a post-hoc analysis.
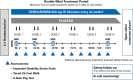
Methods: The proportion of patients with NEPAD (no evidence of progression [NEP; no 12-week confirmed progression of ≥1/≥0.5 points on the Expanded Disability Status Scale if the baseline score was ≤5.5/>5.5 points, respectively; no 12-week confirmed progression of ≥20% on the Timed 25-Foot Walk test and 9-Hole Peg Test], no brain magnetic resonance imaging activity [no new/enlarging T2 lesions and no T1 gadolinium-enhancing lesions], and no protocol-defined relapse) from baseline to week 120 was determined in ocrelizumab- (600 mg; n = 465) and placebo-treated (n = 234) patients.
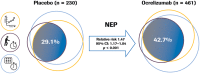
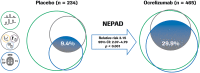
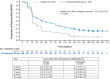
Results: The majority of ORATORIO study patients with PPMS experienced clinical progression or evidence of disease activity. From baseline to week 120, 29.9% and 42.7% ocrelizumab-treated compared to 9.4% and 29.1% placebo-treated patients maintained NEPAD (relative risk [95% confidence interval {CI}], 3.15 [2.07-4.79]; p < 0.001) and NEP (relative risk [95% CI], 1.47 [1.17-1.84]; p < 0.001), respectively. Effects on the individual components of both measures were consistent with the compound outcomes.
Interpretation: Compared to placebo, ocrelizumab enhanced 3-fold the proportion of PPMS patients with no evidence of either progression or inflammatory disease activity. NEPAD may represent a sensitive and meaningful comprehensive measure of disease control in patients with PPMS. Ann Neurol 2018;84:527-536.
© 2018 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures
References
-
- Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol 2014;72(suppl 1):1–5. - PubMed
-
- Wolinsky JS, Narayana PA, O'Connor P, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double‐blind, placebo‐controlled trial. Ann Neurol 2007;61:14–24. - PubMed
-
- Hawker K, O'Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double‐blind placebo‐controlled multicenter trial. Ann Neurol 2009;66:460–471. - PubMed
-
- Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet 2016;387:1075–1084. - PubMed
-
- Balabanov P, Haas M, Elferink A, et al. Addressing the regulatory and scientific challenges in multiple sclerosis—a statement from the EU regulators. Mult Scler 2014;20:1282–1287. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

