Vascular endothelial growth factor activates neural stem cells through epidermal growth factor receptor signal after spinal cord injury
- PMID: 30155986
- PMCID: PMC6488895
- DOI: 10.1111/cns.13056
Vascular endothelial growth factor activates neural stem cells through epidermal growth factor receptor signal after spinal cord injury
Abstract
Aims: Neural stem cells (NSCs) in the adult mammalian spinal cord are activated in response to spinal cord injury (SCI); however, mechanisms modulating this process are not clear. Here, we noticed SCI elevated expression of vascular endothelial growth factor (VEGF) and we aimed to validate the roles of VEGF in NSCs activation after SCI and investigated the related signals during the process.
Methods: In vitro we detected whether VEGF promoted spinal cord NSCs proliferation and investigated the involved signals; In vivo, we injected VEGF into rat spinal cord to check the NSCs activation.
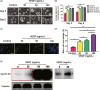
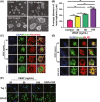
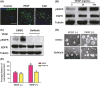
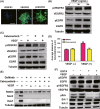
Results: In vitro, VEGF triggered spinal cord NSCs proliferation and maintained self-renewal. Further investigations demonstrated VEGF transactivated epidermal growth factor receptor (EGFR) through VEGF receptor 2 (VEGFR2) to promote spinal cord NSCs proliferation. In vivo, we injected VEGF into spinal cord by laminectomy to confirm the roles of VEGF-VEGFR2-EGFR signals in NSCs activation. VEGF significantly elevated the number of activated NSCs and increased EGFR phosphorylation. In contrast, intraspinal injection of specific inhibitors targeting EGFR and VEGFR2 decreased NSCs activation after SCI. Our results demonstrate that VEGF-VEGFR2-EGFR axis is important for NSCs activation after SCI, providing new insights into the mechanisms of spinal cord NSCs activation postinjury.
Keywords: VEGF receptor 2; epidermal growth factor receptor; neural stem cells activation; spinal cord injury; vascular endothelial growth factor.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Transplantation of Recombinant Vascular Endothelial Growth Factor (VEGF)189-Neural Stem Cells Downregulates Transient Receptor Potential Vanilloid 1 (TRPV1) and Improves Motor Outcome in Spinal Cord Injury.Med Sci Monit. 2018 Feb 21;24:1089-1096. doi: 10.12659/msm.905264. Med Sci Monit. 2018. PMID: 29466323 Free PMC article.
-
Low-energy extracorporeal shock wave therapy promotes vascular endothelial growth factor expression and improves locomotor recovery after spinal cord injury.J Neurosurg. 2014 Dec;121(6):1514-25. doi: 10.3171/2014.8.JNS132562. Epub 2014 Oct 3. J Neurosurg. 2014. PMID: 25280090
-
Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury.J Neurosurg Spine. 2016 Dec;25(6):745-755. doi: 10.3171/2016.4.SPINE15923. Epub 2016 Jul 1. J Neurosurg Spine. 2016. PMID: 27367940
-
Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair.Cells. 2022 Mar 1;11(5):846. doi: 10.3390/cells11050846. Cells. 2022. PMID: 35269466 Free PMC article. Review.
-
A review of vascular endothelial growth factor and its potential to improve functional outcomes following spinal cord injury.Spinal Cord. 2023 Apr;61(4):231-237. doi: 10.1038/s41393-023-00884-4. Epub 2023 Mar 6. Spinal Cord. 2023. PMID: 36879041 Review.
Cited by
-
Effect of Electroacupuncture Stimulation on Proliferation and Differentiation of Endogenous Neural Stem Cells in Rats with Spinal Cord Injury.Mol Neurobiol. 2024 Feb;61(2):635-645. doi: 10.1007/s12035-023-03577-4. Epub 2023 Aug 31. Mol Neurobiol. 2024. PMID: 37650966
-
miR-30b Promotes spinal cord sensory function recovery via the Sema3A/NRP-1/PlexinA1/RhoA/ROCK Pathway.J Cell Mol Med. 2020 Nov;24(21):12285-12297. doi: 10.1111/jcmm.15591. Epub 2020 Sep 25. J Cell Mol Med. 2020. PMID: 32977360 Free PMC article.
-
Neuroprotective Effects of VEGF-A Nanofiber Membrane and FAAH Inhibitor URB597 Against Oxygen-Glucose Deprivation-Induced Ischemic Neuronal Injury.Int J Nanomedicine. 2021 May 27;16:3661-3678. doi: 10.2147/IJN.S307335. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34093011 Free PMC article.
-
Exercise training remodels inguinal white adipose tissue through adaptations in innervation, vascularization, and the extracellular matrix.Cell Rep. 2023 Apr 25;42(4):112392. doi: 10.1016/j.celrep.2023.112392. Epub 2023 Apr 13. Cell Rep. 2023. PMID: 37058410 Free PMC article.
-
Thyroid Hormone and Neural Stem Cells: Repair Potential Following Brain and Spinal Cord Injury.Front Neurosci. 2020 Aug 26;14:875. doi: 10.3389/fnins.2020.00875. eCollection 2020. Front Neurosci. 2020. PMID: 32982671 Free PMC article. Review.
References
-
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707‐1710. - PubMed
-
- Temple S. The development of neural stem cells. Nature. 2001;414(6859):112‐117. - PubMed
-
- McKay R. Stem cells in the central nervous system. Science. 1997;276(5309):66‐71. - PubMed
-
- Xu Y, Kitada M, Yamaguchi M, Dezawa M, Ide C. Increase in bFGF‐responsive neural progenitor population following contusion injury of the adult rodent spinal cord. Neurosci Lett. 2006;397(3):174‐179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

