Applications of tumor chip technology
- PMID: 30156248
- PMCID: PMC6207449
- DOI: 10.1039/c8lc00330k
Applications of tumor chip technology
Abstract
Over the past six decades the inflation-adjusted cost to bring a new drug to market has been increasing constantly and doubles every 9 years - now reaching in excess of $2.5 billion. Overall, the likelihood of FDA approval for a drug (any disease indication) that has entered phase I clinical trials is a mere 9.6%, with the approval rate for oncology far below average at only 5.1%. Lack of efficacy or toxicity is often not revealed until the later stages of clinical trials, despite promising preclinical data. This indicates that the current in vitro systems for drug screening need to be improved for better predictability of in vivo outcomes. Microphysiological systems (MPS), or bioengineered 3D microfluidic tissue and organ constructs that mimic physiological and pathological processes in vitro, can be leveraged across preclinical research and clinical trial stages to transform drug development and clinical management for a range of diseases. Here we review the current state-of-the-art in 3D tissue-engineering models developed for cancer research, with a focus on tumor-on-a-chip, or tumor chip, models. From our viewpoint, tumor chip systems can advance innovative medicine to ameliorate the high failure rates in anti-cancer drug development and clinical treatment.
Conflict of interest statement
7 Conflicts
CCWH is co-founder of Kino Biosciences Inc., a company that develops MPS.
Figures



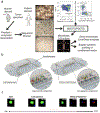
References
-
- Siegel RL, Miller KD and Jemal A, CA. Cancer J. Clin, 2018, 68, 7–30. - PubMed
-
- Kola I and Landis J, Nat. Rev. Drug Discov, 2004, 3, 711–716. - PubMed
-
- Smietana K, Siatkowski M and Møller M, Nat. Rev. Drug Discov, 2016, 15, 379–380. - PubMed
-
- Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, Pairaudeau G, Pennie WD, Pickett SD,Wang J, Wallace O and Weir A, Nat. Rev. Drug Discov, 2015, 14, 475–486. - PubMed
-
- DiMasi JA, Grabowski HG and Hansen RW, J. Health Econ, 2016, 47, 20–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

