Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: A multicenter, double-blind, randomized phase III study
- PMID: 30156314
- PMCID: PMC6220848
- DOI: 10.1111/1346-8138.14607
Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: A multicenter, double-blind, randomized phase III study
Abstract
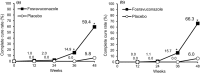
Fosravuconazole L-lysine ethanolate (F-RVCZ) is a prodrug of ravuconazole, a novel triazole antifungal agent, exerting broad and potent antifungal activity. The efficacy and safety of F-RVCZ, compared with a placebo, were investigated in a multicenter, double-blind, randomized study of Japanese onychomycosis patients with 25% or more clinical involvement of the target toenail. Subjects (n = 153) were randomly assigned to receive F-RVCZ (100 mg RVCZ, n = 101) or placebo (n = 52) p.o. once daily for 12 weeks. The primary end-point was the rate of complete cure (clinical cure [0% clinical involvement of the target toenail] plus mycological cure [negative potassium hydroxide examination]) at week 48 (36-week post-treatment visit). Secondary end-points were changes over time in the efficacy and mycological effect of F-RVCZ. Safety was also evaluated. The complete cure rate at week 48 was significantly higher with F-RVCZ (59.4%, 60/101) than the placebo (5.8%, 3/52) in the full analysis set (P < 0.001). The mycological cure rate at week 48 was also significantly higher with F-RVCZ (82.0%, 73/89) than the placebo (20.0%, 10/50, P < 0.001). Regarding safety, adverse events were observed in 83.2% (84/101) and 80.8% (42/52), and adverse drug reactions (ADR) in 23.8% (24/101) and 3.8% (2/52) of F-RVCZ and placebo subjects, respectively. ADR were mild to moderate in severity, with none being serious. F-RVCZ (equivalent to 100 mg ravuconazole) administrated once daily for 12 weeks was more effective than placebo and tolerable in patients with onychomycosis, suggesting it to be a promising drug for onychomycosis treatment.
Keywords: fosravuconazole; onychomycosis; oral antifungal agents; randomized controlled trial; ravuconazole.
© 2018 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Figures





References
-
- Epidemiological Investigation Committee for Human Mycoses in the Japanese Society for Medical Mycology (Chairman and Reporter Yoshihiro Sei) . 2011 Epidemiological survey of dermatomycoses in Japan. Med Mycol J 2015; 56: J129–J135. (in Japanese). - PubMed
-
- Yamaguchi H. Potential of ravuconazole and its prodrugs as the new oral therapeutics for onychomycosis. Med Mycol J 2016; 57: E93–E110. - PubMed
-
- Gupta AK, Leonardi C, Stoltz RR, Pierce PF, Conetta B, Ravuconazole Onychomycosis Group . A phase I/II randomized, double‐blind, placebo‐controlled, dose‐ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. J Eur Acad Dermatol Venereol 2005; 19: 437–443. - PubMed
-
- Yamaguchi H, Ikeda F, Iyoda T, Suzuki M, Mikawa T. In vitro antifungal activity of ravuconazole against isolates of dermatophytes and Candida species from patients with dermatomycoses. Med Mycol J 2014; 55: J157–J163. (in Japanese). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

