Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter
- PMID: 30157189
- PMCID: PMC6114286
- DOI: 10.1371/journal.pone.0200040
Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter
Abstract
Background: Particulate matter (PM) pollutant exposure, which induces oxidative stress and inflammation, and vitamin D insufficiency, which compromises immune regulation, are detrimental in asthma.
Objectives: Mechanistic cell culture experiments were undertaken to ascertain whether vitamin D abrogates PM-induced inflammatory responses of human bronchial epithelial cells (HBECs) through enhancement of antioxidant pathways.
Methods: Transcriptome analysis, PCR and ELISA were undertaken to delineate markers of inflammation and oxidative stress; with comparison of expression in primary HBECs from healthy and asthmatic donors cultured with reference urban PM in the presence/absence of vitamin D.
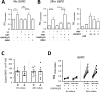
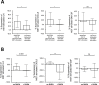
Results: Transcriptome analysis identified over 500 genes significantly perturbed by PM-stimulation, including multiple pro-inflammatory cytokines. Vitamin D altered expression of a subset of these PM-induced genes, including suppressing IL6. Addition of vitamin D suppressed PM-stimulated IL-6 production, although to significantly greater extent in healthy versus asthmatic donor cultures. Vitamin D also differentially affected PM-stimulated GM-CSF, with suppression in healthy HBECs and enhancement in asthmatic cultures. Vitamin D increased HBEC expression of the antioxidant pathway gene G6PD, increased the ratio of reduced to oxidised glutathione, and in PM-stimulated cultures decreased the formation of 8-isoprostane. Pre-treatment with vitamin D decreased CXCL8 and further decreased IL-6 production in PM-stimulated cultures, an effect abrogated by inhibition of G6PD with DHEA, supporting a role for this pathway in the anti-inflammatory actions of vitamin D.
Conclusions: In a study using HBECs from 18 donors, vitamin D enhanced HBEC antioxidant responses and modulated the immune response to PM, suggesting that vitamin D may protect the airways from pathological pollution-induced inflammation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

