Selective ablation of striatal striosomes produces the deregulation of dopamine nigrostriatal pathway
- PMID: 30157254
- PMCID: PMC6114927
- DOI: 10.1371/journal.pone.0203135
Selective ablation of striatal striosomes produces the deregulation of dopamine nigrostriatal pathway
Abstract
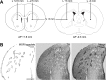
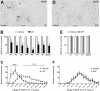
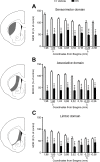
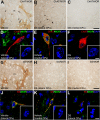
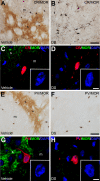
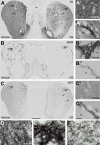
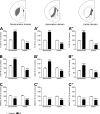
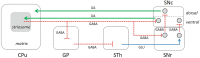
The striatum is a complex structure in which the organization in two compartments (striosomes and matrix) have been defined by their neurochemical profile and their input-output connections. The striosomes receive afferences from the limbic brain areas and send projections to the dopamine neurons of the substantia nigra pars compacta. Thereby, it has been suggested that the striosomes exert a limbic control over the motor function mediated by the surrounding matrix. However, the functionality of the striosomes are not completely understood. To elucidate the role of the striosomes on the regulation of the nigral dopamine neurons, we have induced specific ablation of this compartment by striatal injections of the neurotoxin dermorphin-saporin (DS) and dopamine neurotransmission markers have been analyzed by immunohistochemistry. The degeneration of the striosomes resulted in a nigrostriatal projections imbalance between the two striatal compartments, with an increase of the dopamine neurotransmission in the striosomes and a decrease in the matrix. The present results highlight the key function of the striosomes for the maintenance of the striatal dopamine tone and would contribute to the understanding of their involvement in some neurological disorders such as Huntington's disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–4. - PubMed
-
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Vol. 18, Trends in Neurosciences. 1995. p. 527–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

