Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis
- PMID: 30157388
- PMCID: PMC6172944
- DOI: 10.1056/NEJMoa1803583
Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis
Abstract
Background: There are limited treatments for progressive multiple sclerosis. Ibudilast inhibits several cyclic nucleotide phosphodiesterases, macrophage migration inhibitory factor, and toll-like receptor 4 and can cross the blood-brain barrier, with potential salutary effects in progressive multiple sclerosis.
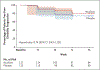
Methods: We enrolled patients with primary or secondary progressive multiple sclerosis in a phase 2 randomized trial of oral ibudilast (≤100 mg daily) or placebo for 96 weeks. The primary efficacy end point was the rate of brain atrophy, as measured by the brain parenchymal fraction (brain size relative to the volume of the outer surface contour of the brain). Major secondary end points included the change in the pyramidal tracts on diffusion tensor imaging, the magnetization transfer ratio in normal-appearing brain tissue, the thickness of the retinal nerve-fiber layer, and cortical atrophy, all measures of tissue damage in multiple sclerosis.
Results: Of 255 patients who underwent randomization, 129 were assigned to ibudilast and 126 to placebo. A total of 53% of the patients in the ibudilast group and 52% of those in the placebo group had primary progressive disease; the others had secondary progressive disease. The rate of change in the brain parenchymal fraction was -0.0010 per year with ibudilast and -0.0019 per year with placebo (difference, 0.0009; 95% confidence interval, 0.00004 to 0.0017; P=0.04), which represents approximately 2.5 ml less brain-tissue loss with ibudilast over a period of 96 weeks. Adverse events with ibudilast included gastrointestinal symptoms, headache, and depression.
Conclusions: In a phase 2 trial involving patients with progressive multiple sclerosis, ibudilast was associated with slower progression of brain atrophy than placebo but was associated with higher rates of gastrointestinal side effects, headache, and depression. (Funded by the National Institute of Neurological Disorders and Stroke and others; NN102/SPRINT-MS ClinicalTrials.gov number, NCT01982942 .).
Figures

References
-
- Ocrevus: EPAR product information. London: European Medicines Agency, 2018. (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_asses...).
-
- Ruiz-Pérez D, Benito J, Polo G, et al. The effects of the toll-like receptor 4 antagonist, ibudilast, on sevoflurane’s minimum alveolar concentration and the delayed remifentanil-induced increase in the minimum alveolar concentration in rats. Anesth Analg 2016; 122: 1370–6. - PubMed
-
- Hagman S, Raunio M, Rossi M, Dastidar P, Elovaara I. Disease-associated inflammatory biomarker profiles in blood in different subtypes of multiple sclerosis: prospective clinical and MRI follow-up study. J Neuroimmunol 2011; 234: 141–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 NS077179/NS/NINDS NIH HHS/United States
- UL1 TR002369/TR/NCATS NIH HHS/United States
- KL2 TR001080/TR/NCATS NIH HHS/United States
- U01 NS082329/NS/NINDS NIH HHS/United States
- U10 NS077265/NS/NINDS NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- UL1 TR001425/TR/NCATS NIH HHS/United States
- UL1 TR002556/TR/NCATS NIH HHS/United States
- U10 NS077260/NS/NINDS NIH HHS/United States
- U01 NS077352/NS/NINDS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U24 NS107128/NS/NINDS NIH HHS/United States
- U24 NS107182/NS/NINDS NIH HHS/United States
- UL1 TR000457/TR/NCATS NIH HHS/United States
- U24 NS107165/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
