Bacterial Factors That Predict Relapse after Tuberculosis Therapy
- PMID: 30157391
- PMCID: PMC6317071
- DOI: 10.1056/NEJMoa1715849
Bacterial Factors That Predict Relapse after Tuberculosis Therapy
Abstract
Background: Approximately 5% of patients with drug-susceptible tuberculosis have a relapse after 6 months of first-line therapy, as do approximately 20% of patients after 4 months of short-course therapy. We postulated that by analyzing pretreatment isolates of Mycobacterium tuberculosis obtained from patients who subsequently had a relapse or were cured, we could determine any correlations between the minimum inhibitory concentration (MIC) of a drug below the standard resistance breakpoint and the relapse risk after treatment.
Methods: Using data from the Tuberculosis Trials Consortium Study 22 (development cohort), we assessed relapse and cure isolates to determine the MIC values of isoniazid and rifampin that were below the standard resistance breakpoint (0.1 μg per milliliter for isoniazid and 1.0 μg per milliliter for rifampin). We combined this analysis with clinical, radiologic, and laboratory data to generate predictive relapse models, which we validated by analyzing data from the DMID 01-009 study (validation cohort).
Results: In the development cohort, the mean (±SD) MIC of isoniazid below the breakpoint was 0.0334±0.0085 μg per milliliter in the relapse group and 0.0286±0.0092 μg per milliliter in the cure group, which represented a higher value in the relapse group by a factor of 1.17 (P=0.02). The corresponding MIC values of rifampin were 0.0695±0.0276 and 0.0453±0.0223 μg per milliliter, respectively, which represented a higher value in the relapse group by a factor of 1.53 (P<0.001). Higher MIC values remained associated with relapse in a multivariable analysis that included other significant between-group differences. In an analysis of receiver-operating-characteristic curves of relapse based on these MIC values, the area under the curve (AUC) was 0.779. In the development cohort, the AUC in a multivariable model that included MIC values was 0.875. In the validation cohort, the MIC values either alone or combined with other patient characteristics were also predictive of relapse, with AUC values of 0.964 and 0.929, respectively. The use of a model score for the MIC values of isoniazid and rifampin to achieve 75.0% sensitivity in cross-validation analysis predicted relapse with a specificity of 76.5% in the development cohort and a sensitivity of 70.0% and a specificity of 100% in the validation cohort.
Conclusions: In pretreatment isolates of M. tuberculosis with decrements of MIC values of isoniazid or rifampin below standard resistance breakpoints, higher MIC values were associated with a greater risk of relapse than lower MIC values. (Funded by the National Institute of Allergy and Infectious Diseases.).
Figures

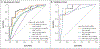

Comment in
-
Of MICs and Men.N Engl J Med. 2018 Aug 30;379(9):882-883. doi: 10.1056/NEJMe1808564. N Engl J Med. 2018. PMID: 30157402 No abstract available.
-
Bacterial Factors and Relapse after Tuberculosis Therapy.N Engl J Med. 2019 Jan 10;380(2):197. doi: 10.1056/NEJMc1813645. N Engl J Med. 2019. PMID: 30628424 No abstract available.
References
-
- Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis 2003;3:282–7. - PubMed
-
- Merle CS, Fielding K, Sow OB, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med 2014;371:1588–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
