Daphnane diterpenes inhibit the metastatic potential of B16F10 murine melanoma cells in vitro and in vivo
- PMID: 30157785
- PMCID: PMC6116488
- DOI: 10.1186/s12885-018-4693-y
Daphnane diterpenes inhibit the metastatic potential of B16F10 murine melanoma cells in vitro and in vivo
Erratum in
-
Correction to: Daphnane diterpenes inhibit the metastatic potential of B16F10 murine melanoma cells in vitro and in vivo.BMC Cancer. 2018 Sep 18;18(1):902. doi: 10.1186/s12885-018-4805-8. BMC Cancer. 2018. PMID: 30227834 Free PMC article.
Abstract
Background: Melanoma is one of the most invasive and aggressive types of cancer with a very poor prognosis. Surgery remains the most efficient treatment prior melanoma invasion and metastasis formation. However, therapy becomes a challenge once the cancer cells colonized other tissues. At present, there are two main classes of therapies acting with a certain efficiency on metastatic melanoma: immune check point inhibitors (anti-PD1/PDL1) and targeted therapy such as Vemurafenib. Unfortunately, these therapies are not fully responsive, induce resistance and/or generate unwanted side effects. In this respect, it is important to continue to discover new cancer therapeutics. Here, we show that daphnane diterpenes type of compounds can prevent melanoma metastasis by inhibiting metastasis-associated matrix metalloproteinases expression without cytotoxicity.
Methods: Evaluation of the anti-metastasis effect of daphnane diterpenes-rich Thymelaea hirsuta extract (TH) and its bioactive component gnidilatidin was carried out in vitro using B16 murine melanoma cells and in vivo using male C57BL/6 J mice. Global gene expression in B16 cells was done using DNA microarray, validated using real-time PCR, to further understand the effect of daphnane diterpenes, specifically daphnane diterpenoid gnidilatidin.
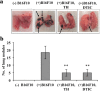
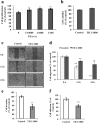
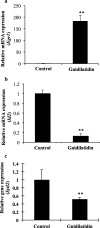
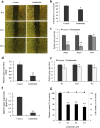
Results: Oral administration of daphnane diterpenes-rich Thymelaea hirsuta extract (TH) suppressed MMP2 and MMP9 expression, decreasing lung tumor in mice injected with B16 murine melanoma cells. Validation of these observations in vitro showed reduced B16 cells migration, adhesion, and invasion. Results of microarray analysis of B16 cells treated with daphnane diterpenoid gnidilatidin from TH revealed an upregulation of tumor suppressor Egr1 while inhibiting metastasis-associated genes Id2 and Sytl2 expression. A downregulation of the melanoma oncogene microphthalmia-associated transcription factor (Mitf) was observed, and most likely caused by the inhibition of Id2, a gene that regulated HLH transcription factors such as MITF and also reported to promote tumor cell migration and invasion.
Conclusions: Daphnane diterpenes have inhibitory effect on the metastatic potential of B16 melanoma cells, and the results of this study provided evidence for their potential for use in the prevention and inhibition of melanoma metastasis.
Keywords: Daphnane diterpenes; Gnidilatidin; Id2; MMPs; Melanoma; Mitf.
Conflict of interest statement
Ethics approval
All the experiments complied with the guidelines of the University of Tsukuba’s Regulation of Animal Experiments and were approved by the University of Tsukuba’s Committee on Animal Care and Use (No. 16–046).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Cancer Statistics. National Cancer Institute at the National Institutes of Health. https://www.cancer.gov/about-cancer/understanding/statistics. Accessed 25 April 2017.
-
- Goding CR. Translation reprogramming key determinant of melanoma invasion. Oncol Times. 2017;39:1–8. doi: 10.1097/01.COT.0000515178.64140.05. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

