Differentially expressed proteins in positive versus negative HNSCC lymph nodes
- PMID: 30157864
- PMCID: PMC6114741
- DOI: 10.1186/s12920-018-0382-6
Differentially expressed proteins in positive versus negative HNSCC lymph nodes
Abstract
Background: Lymph node metastasis is one of the most important prognostic factors in head and neck squamous cell carcinomas (HNSCCs) and critical for delineating their treatment. However, clinical and histological criteria for the diagnosis of nodal status remain limited. In the present study, we aimed to characterize the proteomic profile of lymph node metastasis from HNSCC patients.
Methods: In the present study, we used one- and two-dimensional electrophoresis and mass spectrometry analysis to characterize the proteomic profile of lymph node metastasis from HNSCC.
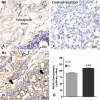
Results: Comparison of metastatic and non-metastatic lymph nodes showed 52 differentially expressed proteins associated with neoplastic development and progression. The results reinforced the idea that tumors from different anatomical subsites have dissimilar behaviors, which may be influenced by micro-environmental factor including the lymphatic network. The expression pattern of heat shock proteins and glycolytic enzymes also suggested an effect of the lymph node environment in controlling tumor growth or in metabolic reprogramming of the metastatic cell. Our study, for the first time, provided direct evidence of annexin A1 overexpression in lymph node metastasis of head and neck cancer, adding information that may be useful for diagnosing aggressive disease.
Conclusions: In brief, this study contributed to our understanding of the metastatic phenotype of HNSCC and provided potential targets for diagnostic in this group of carcinomas.
Keywords: Head and neck carcinoma; Lymph node; Metastasis; Proteomics.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted under approval of the National Committee on Ethics in Research/CONEP (reference number 1763/05, 18/05/2005) and all patients signed informed consent to participate.
Consent for publication
All patients gave written informed consent to publication of their case details, including clinical and pathology report data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

