The inclusion of real world evidence in clinical development planning
- PMID: 30157904
- PMCID: PMC6116448
- DOI: 10.1186/s13063-018-2769-2
The inclusion of real world evidence in clinical development planning
Abstract
Background: When designing studies it is common to search the literature to investigate variability estimates to use in sample size calculations. Proprietary data of previously designed trials in a particular indication are also used to obtain estimates of variability. Estimates of treatment effects are typically obtained from randomised controlled clinical trials (RCTs). Based on the observed estimates of treatment effect, variability and the minimum clinical relevant difference to detect, the sample size for a subsequent trial is estimated. However, data from real world evidence (RWE) studies, such as observational studies and other interventional studies in patients in routine clinical practice, are not widely used in a systematic manner when designing studies. In this paper, we propose a framework for inclusion of RWE in planning of a clinical development programme.
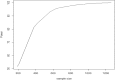
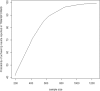
Methods: In our proposed approach, all evidence, from both RCTs and RWE (i.e. from studies in routine clinical practice), available at the time of designing of a new clinical trial is combined in a Bayesian network meta-analysis (NMA). The results can be used to inform the design of the next clinical trial in the programme. The NMA was performed at key milestones, such as at the end of the phase II trial and prior to the design of key phase III studies. To illustrate the methods, we designed an alternative clinical development programme in multiple sclerosis using RWE through clinical trial simulations.
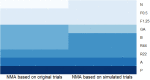
Results: Inclusion of RWE in the NMA and the resulting trial simulations demonstrated that 284 patients per arm were needed to achieve 90% power to detect effects of predetermined size in the TRANSFORMS study. For the FREEDOMS and FREEDOMS II clinical trials, 189 patients per arm were required. Overall there was a reduction in sample size of at least 40% across the three phase III studies, which translated to a time savings of at least 6 months for the undertaking of the fingolimod phase III programme.
Conclusion: The use of RWE resulted in a reduced sample size of the pivotal phase III studies, which led to substantial time savings compared to the approach of sample size calculations without RWE.
Keywords: Clinical development plan; Clinical trial simulation; Negative binomial model; Network meta-analysis; Relapse rate; Sample size.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Design issues in randomized phase II/III trials.J Clin Oncol. 2012 Feb 20;30(6):667-71. doi: 10.1200/JCO.2011.38.5732. Epub 2012 Jan 23. J Clin Oncol. 2012. PMID: 22271475 Free PMC article.
-
Seamlessly expanding a randomized phase II trial to phase III.Biometrics. 2002 Dec;58(4):823-31. doi: 10.1111/j.0006-341x.2002.00823.x. Biometrics. 2002. PMID: 12495136
-
Blinded continuous monitoring in clinical trials with recurrent event endpoints.Pharm Stat. 2019 Jan;18(1):54-64. doi: 10.1002/pst.1907. Epub 2018 Oct 21. Pharm Stat. 2019. PMID: 30345693 Free PMC article.
-
General keynote: clinical trial design.Gynecol Oncol. 2003 Jan;88(1 Pt 2):S114-6; S122-3. doi: 10.1006/gyno.2002.6951. Gynecol Oncol. 2003. PMID: 12586099 Review. No abstract available.
Cited by
-
Framework for the synthesis of non-randomised studies and randomised controlled trials: a guidance on conducting a systematic review and meta-analysis for healthcare decision making.BMJ Evid Based Med. 2022 Apr;27(2):109-119. doi: 10.1136/bmjebm-2020-111493. Epub 2020 Dec 9. BMJ Evid Based Med. 2022. PMID: 33298465 Free PMC article.
-
Reverse Engineering of Digital Measures: Inviting Patients to the Conversation.Digit Biomark. 2023 May 12;7(1):28-44. doi: 10.1159/000530413. eCollection 2023 Jan-Dec. Digit Biomark. 2023. PMID: 37206894 Free PMC article.
-
RWE Framework: An Interactive Visual Tool to Support a Real-World Evidence Study Design.Drugs Real World Outcomes. 2019 Dec;6(4):193-203. doi: 10.1007/s40801-019-00167-6. Drugs Real World Outcomes. 2019. PMID: 31741199 Free PMC article.
-
Methods for the inclusion of real-world evidence in network meta-analysis.BMC Med Res Methodol. 2021 Oct 9;21(1):207. doi: 10.1186/s12874-021-01399-3. BMC Med Res Methodol. 2021. PMID: 34627166 Free PMC article.
-
Impact of Real-World Data on Market Authorization, Reimbursement Decision & Price Negotiation.Ther Innov Regul Sci. 2021 Jan;55(1):228-238. doi: 10.1007/s43441-020-00208-1. Epub 2020 Aug 28. Ther Innov Regul Sci. 2021. PMID: 32857313 Review.
References
-
- Clayton GL, Smith IL, Higgins JP, Mihaylova B, Thorpe B, Cicero R, Lokuge K, Forman JR, Tierney JF, White IR, Sharples LD, Jones HE. The INVEST project: investigating the use of evidence synthesis in the design and analysis of clinical trials. Trials. 2017;18:219–229. doi: 10.1186/s13063-017-1955-y. - DOI - PMC - PubMed
-
- Annemans L, Aristides M, Kubin M. Real-life data: a growing need. ISPOR connections. 2015;13(5):8–12.
-
- Ankarfeldt MZ, Adalsteinsson E, Groenwold RHH, Ali MS, Klungel OH. A systematic literature review on the efficacy-effectiveness gap: comparison of randomized controlled trials and observational studies of glucose lowering drugs. Clin Epidemiol. 2017;9:41–51. doi: 10.2147/CLEP.S121991. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

