A G1-lineage H9N2 virus with oviduct tropism causes chronic pathological changes in the infundibulum and a long-lasting drop in egg production
- PMID: 30157967
- PMCID: PMC6116506
- DOI: 10.1186/s13567-018-0575-1
A G1-lineage H9N2 virus with oviduct tropism causes chronic pathological changes in the infundibulum and a long-lasting drop in egg production
Abstract
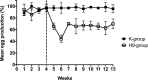
Since 1997, G1-lineage H9N2 avian influenza viruses have been circulating in Asia and later on in the Middle East, and they have been associated to mild respiratory disease, drops in egg production and moderate mortality in chickens, in particular in the presence of concurrent infections. In this study, we investigated the importance of the G1-lineage H9N2 A/chicken/Israel/1163/2011 virus as a primary pathogen in layers, analyzing its tropism and binding affinity for the oviduct tissues, and investigating the long-term impact on egg production. Besides causing a mild respiratory infection, the virus replicated in the oviduct of 60% of the hens causing different degrees of salpingitis throughout the organ, in particular at the level of the infundibulum, where the detection of the virus was associated with severe heterophilic infiltrate, and necrosis of the epithelium. Binding affinity assays confirmed that the infundibulum was the most receptive region of the oviduct. The drop in egg production was at its peek at 2 weeks post-infection (pi) (60% decrease) and continued up to 80 days pi (35% decrease). On day 80 pi, non-laying birds showed egg yolk peritonitis, and histopathological analyses described profound alteration of the infundibulum architecture, duct ectasia and thinning of the epithelium, while the rest of the oviduct and ovary appeared normal. Our results show that this H9N2 virus is a primary pathogen in layer hens, and that its replication in the infundibulum is responsible for acute and chronic lesions that limits the effective functionality of the oviduct, compromising the commercial life of birds.
Figures





References
-
- Swayne DE. Animal influenza. Hoboken: Wiley; 2016.
-
- Fusaro A, Monne I, Salviato A, Valastro V, Schivo A, Amarin NM, Gonzalez C, Ismail MM, Al-Ankari A-R, Al-Blowi MH, Khan OA, Maken Ali AS, Hedayati A, Garcia Garcia J, Ziay GM, Shoushtari A, Al Qahtani KN, Capua I, Holmes EC, Cattoli G. Phylogeography and evolutionary history of reassortant H9N2 viruses with potential human health implications. J Virol. 2011;85:8413–8421. doi: 10.1128/JVI.00219-11. - DOI - PMC - PubMed
-
- Aouini R, Laamiri N, Ghram A. Novel gene mutations in Tunisian isolate of avian H9N2 influenza virus. J Vet Sci Technol. 2016;8:1–9. doi: 10.4172/2157-7579.1000405. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

