One month of hyperglycemia alters spectral responses of the zebrafish photopic electroretinogram
- PMID: 30158110
- PMCID: PMC6215424
- DOI: 10.1242/dmm.035220
One month of hyperglycemia alters spectral responses of the zebrafish photopic electroretinogram
Abstract
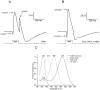
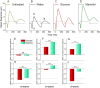
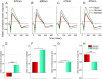
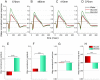
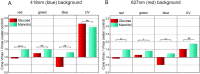
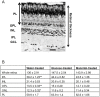
Prolonged hyperglycemia can alter retinal function, ultimately resulting in blindness. Adult zebrafish adults exposed to alternating conditions of 2% glucose/0% glucose display a 3× increase in blood sugar levels. After 4 weeks of treatment, electroretinograms (ERGs) were recorded from isolated, perfused, in vitro eyecups. Control animals were exposed to alternating 2% mannitol/0% mannitol (osmotic control) or to alternating water (0% glucose/0% glucose; handling control). Two types of ERGs were recorded: (1) native ERGs measured using white-light stimuli and medium without synaptic blockers; and (2) spectral ERGs measured with an AMPA/kainate receptor antagonist, isolating photoreceptor-to-ON-bipolar-cell synapses, and a spectral protocol that separated red (R), green (G), blue (B) and UV cone signals. Retinas were evaluated for changes in layer thickness and for the inflammatory markers GFAP and Nf-κB (RelA or p65). In native ERGs, hyperglycemic b- and d-waves were lower in amplitude than the b- and d-waves of mannitol controls. Alteration of waveshape became severe, with b-waves becoming more transient and ERG responses showing more PIII-like (a-wave) characteristics. For spectral ERGs, waveshape appeared similar in all treatment groups. However, a1- and b2-wave implicit times were significantly longer, and amplitudes were significantly reduced, in response to hyperglycemic treatment, owing to the functional reduction in signals from R, G and B cones. Nf-κB increased significantly in hyperglycemic retinas, but the increase in GFAP was not significant and retinal layer thickness was unaffected. Thus, prolonged hyperglycemia triggers an inflammatory response and functional deficits localized to specific cone types, indicating the rapid onset of neural complications in the zebrafish model of diabetic retinopathy.
Keywords: A-wave; B-wave; Diabetes; Glucose; ON bipolar; Outer retina.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
A spectral model for signal elements isolated from zebrafish photopic electroretinogram.Vis Neurosci. 2009 Jul-Aug;26(4):349-63. doi: 10.1017/S0952523809990113. Epub 2009 Sep 2. Vis Neurosci. 2009. PMID: 19723365 Free PMC article.
-
Toluene inhalation exposure for 13 weeks causes persistent changes in electroretinograms of Long-Evans rats.Neurotoxicology. 2016 Mar;53:257-270. doi: 10.1016/j.neuro.2016.02.008. Epub 2016 Feb 17. Neurotoxicology. 2016. PMID: 26899397 Free PMC article.
-
Retinal bipolar cell input mechanisms in giant danio. I. Electroretinographic analysis.J Neurophysiol. 2005 Jan;93(1):84-93. doi: 10.1152/jn.00259.2004. Epub 2004 Jun 30. J Neurophysiol. 2005. PMID: 15229213
-
[Animal models of human retinal and optic nerve diseases analysed using electroretinography].Nippon Ganka Gakkai Zasshi. 2010 Mar;114(3):248-78, discussion 279. Nippon Ganka Gakkai Zasshi. 2010. PMID: 20387538 Review. Japanese.
-
Color Processing in Zebrafish Retina.Front Cell Neurosci. 2018 Oct 3;12:327. doi: 10.3389/fncel.2018.00327. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30337857 Free PMC article. Review.
Cited by
-
Scientometric analysis and historical review of diabetic encephalopathy research: Trends and hotspots (2004-2023).World J Diabetes. 2025 May 15;16(5):91200. doi: 10.4239/wjd.v16.i5.91200. World J Diabetes. 2025. PMID: 40487602 Free PMC article.
-
Are Hyperglycemia-Induced Changes in the Retina Associated with Diabetes-Correlated Changes in the Brain? A Review from Zebrafish and Rodent Type 2 Diabetes Models.Biology (Basel). 2024 Jun 27;13(7):477. doi: 10.3390/biology13070477. Biology (Basel). 2024. PMID: 39056672 Free PMC article. Review.
-
Advancing Diabetic Retinopathy Research: Analysis of the Neurovascular Unit in Zebrafish.Cells. 2021 May 25;10(6):1313. doi: 10.3390/cells10061313. Cells. 2021. PMID: 34070439 Free PMC article. Review.
-
Diabetic photoreceptors: Mechanisms underlying changes in structure and function.Vis Neurosci. 2020 Oct 6;37:E008. doi: 10.1017/S0952523820000097. Vis Neurosci. 2020. PMID: 33019947 Free PMC article. Review.
-
Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery.Pharmaceuticals (Basel). 2021 Jul 25;14(8):716. doi: 10.3390/ph14080716. Pharmaceuticals (Basel). 2021. PMID: 34451814 Free PMC article. Review.
References
-
- Acerete L., Balasch J. C., Espinosa E., Josa A. and Tort L. (2004). Physiological responses in Eurasian perch (Perca fluviatilis, L.) subjected to stress by transport and handling. Aquaculture 237, 167-178. 10.1016/j.aquaculture.2004.03.018 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

