Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability
- PMID: 30158152
- PMCID: PMC6454550
- DOI: 10.1126/scitranslmed.aam6474
Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability
Abstract
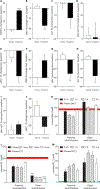
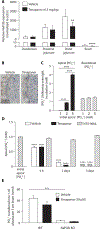
Hyperphosphatemia is common in patients with chronic kidney disease and is increasingly associated with poor clinical outcomes. Current management of hyperphosphatemia with dietary restriction and oral phosphate binders often proves inadequate. Tenapanor, a minimally absorbed, small-molecule inhibitor of the sodium/hydrogen exchanger isoform 3 (NHE3), acts locally in the gastrointestinal tract to inhibit sodium absorption. Because tenapanor also reduces intestinal phosphate absorption, it may have potential as a therapy for hyperphosphatemia. We investigated the mechanism by which tenapanor reduces gastrointestinal phosphate uptake, using in vivo studies in rodents and translational experiments on human small intestinal stem cell-derived enteroid monolayers to model ion transport physiology. We found that tenapanor produces its effect by modulating tight junctions, which increases transepithelial electrical resistance (TEER) and reduces permeability to phosphate, reducing paracellular phosphate absorption. NHE3-deficient monolayers mimicked the phosphate phenotype of tenapanor treatment, and tenapanor did not affect TEER or phosphate flux in the absence of NHE3. Tenapanor also prevents active transcellular phosphate absorption compensation by decreasing the expression of NaPi2b, the major active intestinal phosphate transporter. In healthy human volunteers, tenapanor (15 mg, given twice daily for 4 days) increased stool phosphorus and decreased urinary phosphorus excretion. We determined that tenapanor reduces intestinal phosphate absorption predominantly through reduction of passive paracellular phosphate flux, an effect mediated exclusively via on-target NHE3 inhibition.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures





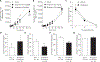
References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group, KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. Suppl, S1–S130 (2009). - PubMed
-
- Walton J, Gray TK, Absorption of inorganic phosphate in the human small intestine. Clin. Sci 56, 407–412 (1979). - PubMed
-
- Davis GR, Zerwekh JE, Parker TF, Krejs GJ, Pak CY, Fordtran JS, Absorption of phosphate in the jejunum of patients with chronic renal failure before and after correction of vitamin D deficiency. Gastroenterology 85, 908–916 (1983). - PubMed
-
- Aloia JF, Yeh JK, Effect of hypophysectomy on intestinal phosphate absorption in rats. Bone 6, 73–77 (1985). - PubMed
-
- Williams KB, DeLuca HF, Characterization of intestinal phosphate absorption using a novel in vivo method. Am. J. Physiol. Endocrinol. Metab 292, E1917–E1921 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

