Human Pathogenic Entomophthorales
- PMID: 30158298
- PMCID: PMC6148186
- DOI: 10.1128/CMR.00014-18
Human Pathogenic Entomophthorales
Abstract
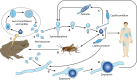
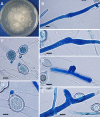
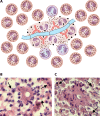
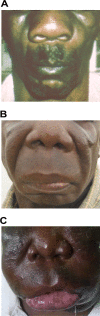
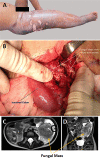
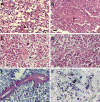
The pathogenic entomophthoralean fungi cause infection in insects and mammalian hosts. Basidiobolus and Conidiobolus species can be found in soil and insect, reptile, and amphibian droppings in tropical and subtropical areas. The life cycles of these fungi occur in these environments where infecting sticky conidia are developed. The infection is acquired by insect bite or contact with contaminated environments through open skin. Conidiobolus coronatus typically causes chronic rhinofacial disease in immunocompetent hosts, whereas some Conidiobolus species can be found in immunocompromised patients. Basidiobolus ranarum infection is restricted to subcutaneous tissues but may be involved in intestinal and disseminated infections. Its early diagnosis remains challenging due to clinical similarities to other intestinal diseases. Infected tissues characteristically display eosinophilic granulomas with the Splendore-Höeppli phenomenon. However, in immunocompromised patients, the above-mentioned inflammatory reaction is absent. Laboratory diagnosis includes wet mount, culture serological assays, and molecular methodologies. The management of entomophthoralean fungi relies on traditional antifungal therapies, such as potassium iodide (KI), amphotericin B, itraconazole, and ketoconazole, and surgery. These species are intrinsically resistant to some antifungals, prompting physicians to experiment with combinations of therapies. Research is needed to investigate the immunology of entomophthoralean fungi in infected hosts. The absence of an animal model and lack of funding severely limit research on these fungi.
Keywords: Basidiobolus; Conidiobolus; Entomophthorales; Entomophthoramycota; basidiobolomycosis; conidiobolomycosis; entomophthoramycosis; rhinoconidiobolomycosis; rhinoentomophthoramycosis.
Copyright © 2018 American Society for Microbiology.
Figures
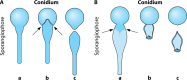

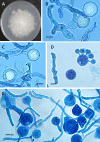
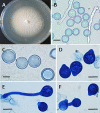
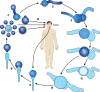


References
-
- Blaché R, Destombes P, Nazimoff O. 1961. Mycoses sous-cutanées nouvelles an Sud-Cameroun. Bull Soc Path Exot 54:56–63.
-
- Brefeld O. 1884. Conidiobolus utricolosis und minor. Untersuchungen aus dem Gesamtgebiete der. Mykologie 4:75–78.
-
- Couch JN. 1939. A new Conidiobolus with sexual reproduction. Am J Bot 26:119–130. doi: 10.1002/j.1537-2197.1939.tb12878.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

