The Virology of Taterapox Virus In Vitro
- PMID: 30158437
- PMCID: PMC6163509
- DOI: 10.3390/v10090463
The Virology of Taterapox Virus In Vitro
Abstract
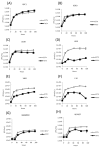
Taterapox virus (TATV) is phylogenetically the closest related virus to variola-the etiological agent of smallpox. Despite the similarity, few studies have evaluated the virus. In vivo, TATV can infect several animals but produces an inapparent infection in wild-type mice; however, TATV does cause morbidity and mortality in some immunocompromised strains. We employed in vitro techniques to compare TATV to ectromelia (ECTV) and vaccinia (VACV) viruses. Both ECTV and TATV replicate efficiently in primate cell lines but TATV replicates poorly in murine cells lines. Furthermore, TATV induces cytopathic effects, but to a lesser extent than ECTV, and changes cytoskeletal networks differently than both ECTV and VACV. Bioinformatic studies revealed differences in several immunomodulator open reading frames that could contribute to the reduced virulence of TATV, which were supported by in vitro cytokine assays.
Keywords: ectromelia; gerbil; orthopoxvirus; smallpox; taterapox; vaccinia; variola.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Chen N., Bellone C.J., Schriewer J., Owens G., Fredrickson T., Parker S., Buller R.M. Poxvirus interleukin-4 expression overcomes inherent resistance and vaccine-induced immunity: Pathogenesis, prophylaxis, and antiviral therapy. Virology. 2011;409:328–337. doi: 10.1016/j.virol.2010.10.021. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

