Updates on Old and Weary Haematopoiesis
- PMID: 30158459
- PMCID: PMC6163425
- DOI: 10.3390/ijms19092567
Updates on Old and Weary Haematopoiesis
Abstract
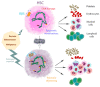
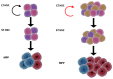
Blood formation, or haematopoiesis, originates from haematopoietic stem cells (HSCs), whose functions and maintenance are regulated in both cell- and cell non-autonomous ways. The surroundings of HSCs in the bone marrow create a specific niche or microenvironment where HSCs nest that allows them to retain their unique characteristics and respond rapidly to external stimuli. Ageing is accompanied by reduced regenerative capacity of the organism affecting all systems, due to the progressive decline of stem cell functions. This includes blood and HSCs, which contributes to age-related haematological disorders, anaemia, and immunosenescence, among others. Furthermore, chronological ageing is characterised by myeloid and platelet HSC skewing, inflammageing, and expanded clonal haematopoiesis, which may be the result of the accumulation of preleukaemic lesions in HSCs. Intriguingly, haematological malignancies such as acute myeloid leukaemia have a high incidence among elderly patients, yet not all individuals with clonal haematopoiesis develop leukaemias. Here, we discuss recent work on these aspects, their potential underlying molecular mechanisms, and the first cues linking age-related changes in the HSC niche to poor HSC maintenance. Future work is needed for a better understanding of haematopoiesis during ageing. This field may open new avenues for HSC rejuvenation and therapeutic strategies in the elderly.
Keywords: ageing; bone marrow; clonal haematopoiesis; haematopoiesis; haematopoietic stem cell niche; inflammageing; leukaemia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pietras E.M., Reynaud D., Kang Y.A., Carlin D., Calero-Nieto F.J., Leavitt A.D., Stuart J.M., Gottgens B., Passegue E. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions. Cell Stem Cell. 2015;17:35–46. doi: 10.1016/j.stem.2015.05.003. - DOI - PMC - PubMed
-
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. - PubMed
Publication types
MeSH terms
Grants and funding
- 2014/5668/Northern Norway Regional Health Authority, the University Hospital of Northern Norway (UNN) and UiT The Arctic University of Norway (UiT)
- Stem Cell Program, 247596/Young Research Talent grants from the Research Council of Norway
- FRIPRO Program, 250901/Young Research Talent grants from the Research Council of Norway
- 6765150/Norwegian Cancer Society
- HNF1338-17/Northern Norway Regional Health Authority
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

