Regulation of Energy Expenditure and Brown/Beige Thermogenic Activity by Interleukins: New Roles for Old Actors
- PMID: 30158466
- PMCID: PMC6164446
- DOI: 10.3390/ijms19092569
Regulation of Energy Expenditure and Brown/Beige Thermogenic Activity by Interleukins: New Roles for Old Actors
Abstract
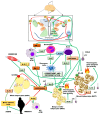
Obesity rates and the burden of metabolic associated diseases are escalating worldwide Energy burning brown and inducible beige adipocytes in human adipose tissues (ATs) have attracted considerable attention due to their therapeutic potential to counteract the deleterious metabolic effects of nutritional overload and overweight. Recent research has highlighted the relevance of resident and recruited ATs immune cell populations and their signalling mediators, cytokines, as modulators of the thermogenic activity of brown and beige ATs. In this review, we first provide an overview of the developmental, cellular and functional heterogeneity of the AT organ, as well as reported molecular switches of its heat-producing machinery. We also discuss the key contribution of various interleukins signalling pathways to energy and metabolic homeostasis and their roles in the biogenesis and function of brown and beige adipocytes. Besides local actions, attention is also drawn to their influence in the central nervous system (CNS) networks governing energy expenditure.
Keywords: brown and beige adipose tissue; cytokines; energy and metabolic homeostasis; inflammation; interleukins; thermogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Recruitment of Thermogenic Fat: Trigger of Fat Burning.Front Endocrinol (Lausanne). 2021 Jul 22;12:696505. doi: 10.3389/fendo.2021.696505. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34367068 Free PMC article. Review.
-
Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis.J Clin Invest. 2015 Feb;125(2):478-86. doi: 10.1172/JCI78362. Epub 2015 Feb 2. J Clin Invest. 2015. PMID: 25642708 Free PMC article. Review.
-
Adaptive thermogenesis in adipocytes: is beige the new brown?Genes Dev. 2013 Feb 1;27(3):234-50. doi: 10.1101/gad.211649.112. Genes Dev. 2013. PMID: 23388824 Free PMC article. Review.
-
Brown and beige fat: the metabolic function, induction, and therapeutic potential.Front Med. 2015 Jun;9(2):162-72. doi: 10.1007/s11684-015-0382-2. Epub 2015 Jan 8. Front Med. 2015. PMID: 25573295 Review.
-
The evolving view of thermogenic adipocytes - ontogeny, niche and function.Nat Rev Endocrinol. 2021 Dec;17(12):726-744. doi: 10.1038/s41574-021-00562-6. Epub 2021 Oct 8. Nat Rev Endocrinol. 2021. PMID: 34625737 Free PMC article. Review.
Cited by
-
Garlic oil suppresses high-fat diet induced obesity in rats through the upregulation of UCP-1 and the enhancement of energy expenditure.Exp Ther Med. 2020 Feb;19(2):1536-1540. doi: 10.3892/etm.2019.8386. Epub 2019 Dec 27. Exp Ther Med. 2020. PMID: 32010335 Free PMC article.
-
Thymic stromal lymphopoietin induces IL-4/IL-13 from T cells to promote sebum secretion and adipose loss.J Allergy Clin Immunol. 2024 Aug;154(2):480-491. doi: 10.1016/j.jaci.2023.11.923. Epub 2023 Dec 28. J Allergy Clin Immunol. 2024. PMID: 38157943 Free PMC article.
-
Macrophages and brown adipocytes cross-communicate to modulate a thermogenic program following methamphetamine exposure.Int J Hyperthermia. 2020;37(1):1368-1382. doi: 10.1080/02656736.2020.1849822. Int J Hyperthermia. 2020. PMID: 33307890 Free PMC article.
-
White and brown adipose tissue functionality is impaired by fine particulate matter (PM2.5) exposure.J Mol Med (Berl). 2022 May;100(5):665-676. doi: 10.1007/s00109-022-02183-6. Epub 2022 Mar 14. J Mol Med (Berl). 2022. PMID: 35286401 Free PMC article. Review.
-
Role of Personalized Nutrition in Chronic-Degenerative Diseases.Nutrients. 2019 Jul 24;11(8):1707. doi: 10.3390/nu11081707. Nutrients. 2019. PMID: 31344895 Free PMC article. Review.
References
-
- Minihane A.M., Vinoy S., Russell W.R., Baka A., Roche H.M., Tuohy K.M., Teeling J.L., Blaak E.E., Fenech M., Vauzour D., et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015;114:999–1012. doi: 10.1017/S0007114515002093. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

