Reconciling persistent and dynamic hypotheses of working memory coding in prefrontal cortex
- PMID: 30158519
- PMCID: PMC6115433
- DOI: 10.1038/s41467-018-05873-3
Reconciling persistent and dynamic hypotheses of working memory coding in prefrontal cortex
Abstract
Competing accounts propose that working memory (WM) is subserved either by persistent activity in single neurons or by dynamic (time-varying) activity across a neural population. Here, we compare these hypotheses across four regions of prefrontal cortex (PFC) in an oculomotor-delayed-response task, where an intervening cue indicated the reward available for a correct saccade. WM representations were strongest in ventrolateral PFC neurons with higher intrinsic temporal stability (time-constant). At the population-level, although a stable mnemonic state was reached during the delay, this tuning geometry was reversed relative to cue-period selectivity, and was disrupted by the reward cue. Single-neuron analysis revealed many neurons switched to coding reward, rather than maintaining task-relevant spatial selectivity until saccade. These results imply WM is fulfilled by dynamic, population-level activity within high time-constant neurons. Rather than persistent activity supporting stable mnemonic representations that bridge subsequent salient stimuli, PFC neurons may stabilise a dynamic population-level process supporting WM.
Conflict of interest statement
The authors declare no competing interests.
Figures



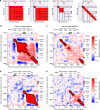
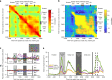

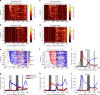
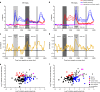
References
Publication types
MeSH terms
Grants and funding
- R21 DA035209/DA/NIDA NIH HHS/United States
- 208789/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- 096689/Z/11/Z/WT_/Wellcome Trust/United Kingdom
- R01-MH097990/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)/International
- R21-DA035209/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

