Revisiting the functional significance of binocular cues for perceiving motion-in-depth
- PMID: 30158523
- PMCID: PMC6115357
- DOI: 10.1038/s41467-018-05918-7
Revisiting the functional significance of binocular cues for perceiving motion-in-depth
Abstract
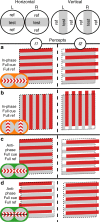
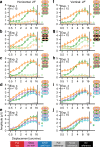
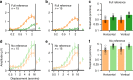
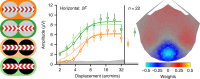
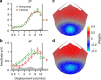
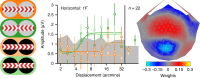
Binocular differencing of spatial cues required for perceiving depth relationships is associated with decreased sensitivity to the corresponding retinal image displacements. However, binocular summation of contrast signals increases sensitivity. Here, we investigated this divergence in sensitivity by making direct neural measurements of responses to suprathreshold motion in human adults and 5-month-old infants using steady-state visually evoked potentials. Interocular differences in retinal image motion generated suppressed response functions and correspondingly elevated perceptual thresholds compared to motion matched between the two eyes. This suppression was of equal strength for horizontal and vertical motion and therefore not specific to the perception of motion-in-depth. Suppression is strongly dependent on the presence of spatial references in the image and highly immature in infants. Suppression appears to be the manifestation of a succession of spatial and interocular opponency operations that occur at an intermediate processing stage either before or in parallel with the extraction of motion-in-depth.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

