Nonproliferative and Proliferative Lesions of the Rat and Mouse Endocrine System
- PMID: 30158740
- PMCID: PMC6108091
- DOI: 10.1293/tox.31.1S
Nonproliferative and Proliferative Lesions of the Rat and Mouse Endocrine System
Abstract
The INHAND (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) Project (www.toxpath.org/inhand.asp) is a joint initiative among the Societies of Toxicological Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP) and North America (STP) to develop an internationally accepted nomenclature for proliferative and nonproliferative lesions in laboratory animals. The purpose of this publication is to provide a standardized nomenclature for classifying microscopic lesions observed in the endocrine organs (pituitary gland, pineal gland, thyroid gland, parathyroid glands, adrenal glands and pancreatic islets) of laboratory rats and mice, with color photomicrographs illustrating examples of the lesions. The standardized nomenclature presented in this document is also available electronically on the internet (http://www.goreni.org/). Sources of material included histopathology databases from government, academia, and industrial laboratories throughout the world. Content includes spontaneous and aging lesions as well as lesions induced by exposure to test materials. A widely accepted and utilized international harmonization of nomenclature for endocrine lesions in laboratory animals will decrease confusion among regulatory and scientific research organizations in different countries and provide a common language to increase and enrich international exchanges of information among toxicologists and pathologists.
Keywords: C cells; adrenal; chief cells; cortical cells; diagnostic pathology; follicular cells; hypophysis; islets; islets of Langerhans; medullary cells; nomenclature; pancreas; parathyroid; pineal; pinealocytes; pituicytes; pituitary; thyroid.
Figures

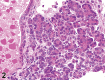



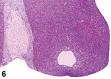

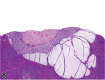


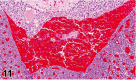


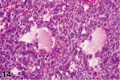





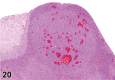





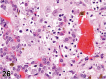




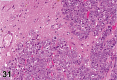

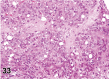


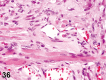


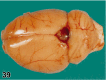
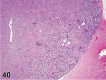





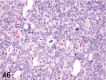
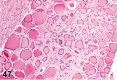
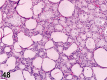



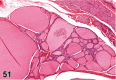
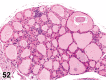
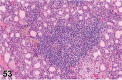

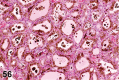
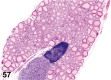

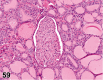



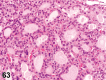
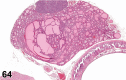
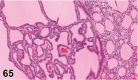
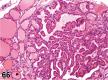

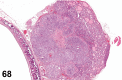
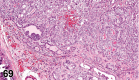


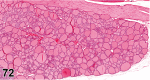
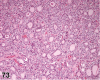

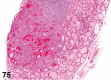
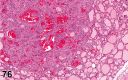



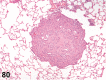










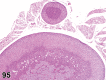






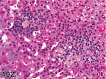
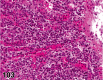


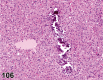



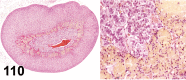




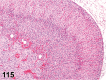



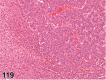




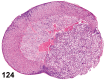





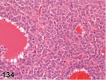

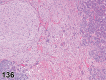
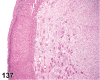
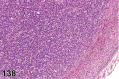
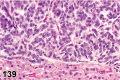
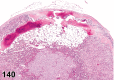
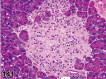

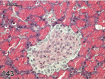












References
-
- Ackermann MR. Cellular and tissue responses to injury. In: Pathologic Basis of Veterinary Disease. Zachary, JF and McGavin, MD (eds). 5th ed. Elsevier, St. Louis, MO. pp. 51–57, 2012.
-
- Adeghate E, Hameed RS, Ponery AS, Tariq S, Sheen RS, Shaffiullah M, and Donáth T. Streptozotocin causes pancreatic beta cell failure via early and sustained biochemical and cellular alterations. Exp Clin Endocrinol Diabetes. 118: 699–707. 2010. - PubMed
-
- Aguzzi A, Wagner EF, Williams RL, and Courtneidge SA. Sympathetic hyperplasia and neuroblastomas in transgenic mice expressing polyoma middle T antigen. New Biol. 2: 533–543. 1990. - PubMed
-
- Aigelsreiter A, Janig E, Stumptner C, Fuchsbichler A, Zatloukal K, and Denk H. How a cell deals with abnormal proteins. Pathogenetic mechanisms in protein aggregation diseases. Pathobiology. 74: 145–158. 2007. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
