A Prospective Case-control Trial to Evaluate and Compare the Efficacy and Safety of Atosiban versus Placebo in In vitro Fertilization-embryo Transfer Program
- PMID: 30158812
- PMCID: PMC6094537
- DOI: 10.4103/jhrs.JHRS_7_17
A Prospective Case-control Trial to Evaluate and Compare the Efficacy and Safety of Atosiban versus Placebo in In vitro Fertilization-embryo Transfer Program
Abstract
Background: Recent developments in assisted reproductive technology focus on potential advances to improve its success rate. Atosiban, a combined oxytocin/vasopressin V1a receptor antagonist, is a novel class of drug involved in basic priming of the uterus for successful implantation during embryo transfer (ET).
Objectives: The objective of this study is to evaluate the efficacy of atosiban (study group) in ET patients in comparison to placebo (control group) regarding implantation rate (IR), clinical pregnancy rate (CPR), and ongoing pregnancy rate and to assess the safety profile of atosiban.
Materials and methods: A total of 320 women undergoing in vitro fertilization-ET at a tertiary care hospital were enrolled in the study. In the study group, atosiban was given as initial intravenous (IV) bolus injection 0.9 ml (6.75 mg), 30 min before ET followed by continuous IV infusion of atosiban. In the control group, placebo (normal saline) was infused at the same rate and dose. Pregnancy was confirmed 14 days after ET by β-human chronic gonadotropin level. IR and CPR were determined by doing transvaginal sonography 3 weeks and 6 weeks postET, respectively.
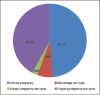
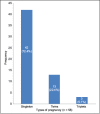
Results: In women with atosiban treatment, the positive pregnancy rate and CPRs were 41.25% and 36.25%, respectively. The IR per embryo transferred was 17.5%. No major side effects of atosiban were noted among enlisted patients. The miscarriage rate and ectopic pregnancy rate were low (12.12% and 4.54%, respectively). Forty-two women had singleton gestation, while twin and triplet pregnancies were encountered in 13 and 3 women, respectively. No congenital anomalies were observed during an antenatal scan at 18-20 weeks in ongoing pregnancies. The positive pregnancy rate, the CPR, and the IR in the control group was 35%, 30%, and 16.5%, respectively, which was significantly lower than the atosiban group.
Conclusion: Atosiban reduces uterine contractions and increases endomyometrial perfusion, both of which have potential benefits regarding improved IRs, CPR, and ongoing pregnancy rates. Atosiban has a good embryonic safety profile.
Keywords: Atosiban; clinical pregnancy rate; embryo transfer; implantation rate; placebo; positive pregnancy rate.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Verhaak CM, Smeenk JM, Evers AW, Kremer JA, Kraaimaat FW, Braat DD, et al. Women's emotional adjustment to IVF: A systematic review of 25 years of research. Hum Reprod Update. 2007;13:27–36. - PubMed
-
- Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21:3036–43. - PubMed
-
- Chou PY, Wu MH, Pan HA, Hung KH, Chang FM. Use of an oxytocin antagonist in in vitro fertilization-embryo transfer for women with repeated implantation failure: A retrospective study. Taiwan J Obstet Gynecol. 2011;50:136–40. - PubMed
-
- Steinwall M, Hansson S, Bossmar T, Larsson I, Pilka R, Akerlund M, et al. Oxytocin mRNA content in the endometrium of non-pregnant women. BJOG. 2004;111:266–70. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical