WWOX Phosphorylation, Signaling, and Role in Neurodegeneration
- PMID: 30158849
- PMCID: PMC6104168
- DOI: 10.3389/fnins.2018.00563
WWOX Phosphorylation, Signaling, and Role in Neurodegeneration
Abstract
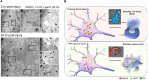
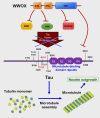
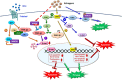
Homozygous null mutation of tumor suppressor WWOX/Wwox gene leads to severe neural diseases, metabolic disorders and early death in the newborns of humans, mice and rats. WWOX is frequently downregulated in the hippocampi of patients with Alzheimer's disease (AD). In vitro analysis revealed that knockdown of WWOX protein in neuroblastoma cells results in aggregation of TRAPPC6AΔ, TIAF1, amyloid β, and Tau in a sequential manner. Indeed, TRAPPC6AΔ and TIAF1, but not tau and amyloid β, aggregates are present in the brains of healthy mid-aged individuals. It is reasonable to assume that very slow activation of a protein aggregation cascade starts sequentially with TRAPPC6AΔ and TIAF1 aggregation at mid-ages, then caspase activation and APP de-phosphorylation and degradation, and final accumulation of amyloid β and Tau aggregates in the brains at greater than 70 years old. WWOX binds Tau-hyperphosphorylating enzymes (e.g., GSK-3β) and blocks their functions, thereby supporting neuronal survival and differentiation. As a neuronal protective hormone, 17β-estradiol (E2) binds WWOX at an NSYK motif in the C-terminal SDR (short-chain alcohol dehydrogenase/reductase) domain. In this review, we discuss how WWOX and E2 block protein aggregation during neurodegeneration, and how a 31-amino-acid zinc finger-like Zfra peptide restores memory loss in mice.
Keywords: 17β-estradiol; TIAF1; TRAPPC6AΔ; WWOX; Zfra; neurodegeneration; sex steroid hormone receptor.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

