Early Disruption of the Microbiome Leading to Decreased Antioxidant Capacity and Epigenetic Changes: Implications for the Rise in Autism
- PMID: 30158857
- PMCID: PMC6104136
- DOI: 10.3389/fncel.2018.00256
Early Disruption of the Microbiome Leading to Decreased Antioxidant Capacity and Epigenetic Changes: Implications for the Rise in Autism
Abstract
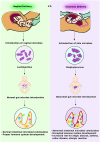
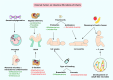
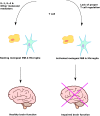
Currently, 1 out of every 59 children in the United States is diagnosed with autism. While initial research to find the possible causes for autism were mostly focused on the genome, more recent studies indicate a significant role for epigenetic regulation of gene expression and the microbiome. In this review article, we examine the connections between early disruption of the developing microbiome and gastrointestinal tract function, with particular regard to susceptibility to autism. The biological mechanisms that accompany individuals with autism are reviewed in this manuscript including immune system dysregulation, inflammation, oxidative stress, metabolic and methylation abnormalities as well as gastrointestinal distress. We propose that these autism-associated biological mechanisms may be caused and/or sustained by dysbiosis, an alteration to the composition of resident commensal communities relative to the community found in healthy individuals and its redox and epigenetic consequences, changes that in part can be due to early use and over-use of antibiotics across generations. Further studies are warranted to clarify the contribution of oxidative stress and gut microbiome in the pathophysiology of autism. A better understanding of the microbiome and gastrointestinal tract in relation to autism will provide promising new opportunities to develop novel treatment modalities.
Keywords: autism; dysbiosis; epigenetics; gut microbiota; oxidative stress.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

