The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin
- PMID: 30158938
- PMCID: PMC6104123
- DOI: 10.3389/fimmu.2018.01904
The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin
Abstract
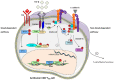
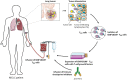
Cancer immunotherapy is aimed at stimulating tumor-specific cytotoxic T lymphocytes and their subsequent trafficking so that they may reach, and persist in, the tumor microenvironment, recognizing and eliminating malignant target cells. Thus, characterization of the phenotype and effector functions of CD8+ T lymphocytes infiltrating human solid tumors is essential for better understanding and manipulating the local antitumor immune response, and for defining their contribution to the success of current cancer immunotherapy approaches. Accumulating evidence indicates that a substantial subpopulation of CD3+CD8+ tumor-infiltrating lymphocytes are tissue resident memory T (TRM) cells, and is emerging as an activated tumor-specific T-cell subset. These TRM cells accumulate in various human cancer tissues, including non-small-cell lung carcinoma (NSCLC), ovarian and breast cancers, and are defined by expression of CD103 [αE(CD103)β7] and/or CD49a [α1(CD49a)β1] integrins, along with C-type lectin CD69, which most likely contribute to their residency characteristic. CD103 binds to the epithelial cell marker E-cadherin, thereby promoting retention of TRM cells in epithelial tumor islets and maturation of cytotoxic immune synapse with specific cancer cells, resulting in T-cell receptor (TCR)-dependent target cell killing. Moreover, CD103 integrin triggers bidirectional signaling events that cooperate with TCR signals to enable T-cell migration and optimal cytokine production. Remarkably, TRM cells infiltrating human NSCLC tumors also express inhibitory receptors such as programmed cell death-1, the neutralization of which, with blocking antibodies, enhances CD103-dependent TCR-mediated cytotoxicity toward autologous cancer cells. Thus, accumulation of TRM cells at the tumor site explains the more favorable clinical outcome, and might be associated with the success of immune checkpoint blockade in a fraction of cancer patients.
Keywords: CD103 integrin; CD8 tissue resident memory T (TRM) cells; cancer immunotherapy; cytotoxic T lymphocytes; onco-immunology.
Figures
References
-
- Sela U, Mauermann N, Hershkoviz R, Zinger H, Dayan M, Cahalon L, et al. The inhibition of autoreactive T cell functions by a peptide based on the CDR1 of an anti-DNA autoantibody is via TGF-beta-mediated suppression of LFA-1 and CD44 expression and function. J Immunol (2005) 175(11):7255–63.10.4049/jimmunol.175.11.7255 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

