Improving agroinfiltration-based transient gene expression in Nicotiana benthamiana
- PMID: 30159002
- PMCID: PMC6109318
- DOI: 10.1186/s13007-018-0343-2
Improving agroinfiltration-based transient gene expression in Nicotiana benthamiana
Abstract
Background: Agroinfiltration is a simple and effective method of delivering transgenes into plant cells for the rapid production of recombinant proteins and has become the preferred transient expression platform to manufacture biologics in plants. Despite its popularity, few studies have sought to improve the efficiency of agroinfiltration to further increase protein yields. This study aimed to increase agroinfiltration-based transient gene expression in Nicotiana benthamiana by improving all levels of transgenesis.
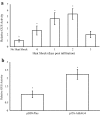
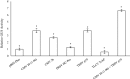
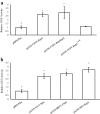
Results: Using the benchmark pEAQ-HT deconstructed virus vector system and the GUS reporter enzyme, physical, chemical, and molecular features were independently assessed for their ability to enhance Agrobacterium-mediated transformation and improve protein production capacities. Optimal Agrobacterium strain, cell culture density and co-cultivation time for maximal transient GUS (β-glucuronidase) expression were established. The effects of chemical additives in the liquid infiltration media were investigated and acetosyringone (500 μM), the antioxidant lipoic acid (5 μM), and a surfactant Pluronic F-68 (0.002%) were all shown to significantly increase transgene expression. Gene products known to suppress post-transcriptional gene silencing, activate cell cycle progression and confer stress tolerance were also assessed by co-expression. A simple 37 °C heat shock to plants, 1-2 days post infiltration, was shown to dramatically increase GUS reporter levels. By combining the most effective features, a dual vector delivery system was developed that provided approximately 3.5-fold higher levels of absolute GUS protein compared to the pEAQ-HT platform.
Conclusions: In this paper, different strategies were assessed and optimised with the aim of increasing plant-made protein capacities in Nicotiana benthamiana using agroinfiltration. Chemical additives, heat shock and the co-expression of genes known to suppress stress and gene silencing or stimulate cell cycle progression were all proven to increase agroinfiltration-based transient gene expression. By combining the most effective of these elements a novel expression platform was developed capable of producing plant-made protein at a significantly higher level than a benchmark hyper-expression system.
Keywords: Agroinfiltration; Hyperexpression; Nicotiana benthamiana; Transient; pEAQ-HT.
Figures



References
-
- Mondal T, Bhattacharya A, Ahuja P, Chand P. Transgenic tea [Camellia sinensis (L.) O. Kuntze cv. Kangra Jat] plants obtained by Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep. 2001;20:712–720. doi: 10.1007/s002990100382. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

