Exosomes Derived from miR-214-Enriched Bone Marrow-Derived Mesenchymal Stem Cells Regulate Oxidative Damage in Cardiac Stem Cells by Targeting CaMKII
- PMID: 30159114
- PMCID: PMC6109555
- DOI: 10.1155/2018/4971261
Exosomes Derived from miR-214-Enriched Bone Marrow-Derived Mesenchymal Stem Cells Regulate Oxidative Damage in Cardiac Stem Cells by Targeting CaMKII
Abstract
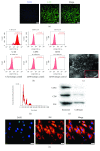
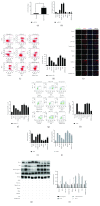
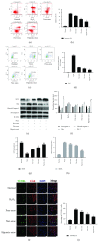
Cardiac stem cells (CSCs) have emerged as one of the most promising stem cells for cardiac protection. Recently, exosomes from bone marrow-derived mesenchymal stem cells (BMSCs) have been found to facilitate cell proliferation and survival by transporting various bioactive molecules, including microRNAs (miRs). In this study, we found that BMSC-derived exosomes (BMSC-exos) significantly decreased apoptosis rates and reactive oxygen species (ROS) production in CSCs after oxidative stress injury. Moreover, a stronger effect was induced by exosomes collected from BMSCs cultured under hypoxic conditions (Hypoxic-exos) than those collected from BMSCs cultured under normal conditions (Nor-exos). We also observed greater miR-214 enrichment in Hypoxic-exos than in Nor-exos. In addition, a miR-214 inhibitor or mimics added to modulate miR-214 levels in BMSC-exos revealed that exosomes from miR-214-depleted BMSCs partially reversed the effects of hypoxia-induced exosomes on oxidative damage in CSCs. These data further confirmed that miR-214 is the main effector molecule in BMSC-exos that protects CSCs from oxidative damage. miR-214 mimic and inhibitor transfection assays verified that CaMKII is a target gene of miR-214 in CSCs, with exosome-pretreated CSCs exhibiting increased miR-214 levels but decreased CaMKII levels. Therefore, the miR-214/CaMKII axis regulates oxidative stress-related injury in CSCs, such as apoptosis, calcium homeostasis disequilibrium, and excessive ROS accumulation. Collectively, these findings suggest that BMSCs release miR-214-containing exosomes to suppress oxidative stress injury in CSCs through CaMKII silencing.
Figures



Similar articles
-
Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis.PLoS One. 2018 Feb 14;13(2):e0191616. doi: 10.1371/journal.pone.0191616. eCollection 2018. PLoS One. 2018. PMID: 29444190 Free PMC article.
-
Bone marrow mesenchymal stem cells-derived exosomes promote spinal cord injury repair through the miR-497-5p/TXNIP/NLRP3 axis.J Mol Histol. 2024 Nov 29;56(1):16. doi: 10.1007/s10735-024-10289-z. J Mol Histol. 2024. PMID: 39611985
-
Bone marrow mesenchymal stem cell-derived exosomes alleviate hyperoxia-induced lung injury via the manipulation of microRNA-425.Arch Biochem Biophys. 2021 Jan 15;697:108712. doi: 10.1016/j.abb.2020.108712. Epub 2020 Nov 29. Arch Biochem Biophys. 2021. PMID: 33264631
-
Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease.Atherosclerosis. 2019 Jun;285:1-9. doi: 10.1016/j.atherosclerosis.2019.03.016. Epub 2019 Mar 23. Atherosclerosis. 2019. PMID: 30939341 Review.
-
Role of cardiac progenitor cell-derived exosome-mediated microRNA-210 in cardiovascular disease.J Cell Mol Med. 2019 Nov;23(11):7124-7131. doi: 10.1111/jcmm.14562. Epub 2019 Sep 26. J Cell Mol Med. 2019. PMID: 31557390 Free PMC article. Review.
Cited by
-
Crosstalk between Oxidative Stress and Exosomes.Oxid Med Cell Longev. 2022 Aug 30;2022:3553617. doi: 10.1155/2022/3553617. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36082080 Free PMC article. Review.
-
Normothermic Machine Perfusion Combined with Bone Marrow Mesenchymal Stem Cells Improves the Oxidative Stress Response and Mitochondrial Function in Rat Donation After Circulatory Death Livers.Stem Cells Dev. 2020 Jul 1;29(13):835-852. doi: 10.1089/scd.2019.0301. Epub 2020 May 5. Stem Cells Dev. 2020. PMID: 32253985 Free PMC article.
-
Extracellular vesicles derived from different sources of mesenchymal stem cells: therapeutic effects and translational potential.Cell Biosci. 2020 May 24;10:69. doi: 10.1186/s13578-020-00427-x. eCollection 2020. Cell Biosci. 2020. PMID: 32483483 Free PMC article. Review.
-
The Role of Exosomal Non-Coding RNAs in Coronary Artery Disease.Front Pharmacol. 2020 Dec 8;11:603104. doi: 10.3389/fphar.2020.603104. eCollection 2020. Front Pharmacol. 2020. PMID: 33363474 Free PMC article. Review.
-
Hypoxia-Elicited Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Alleviate Myocardial Infarction by Promoting Angiogenesis through the miR-214/Sufu Pathway.Stem Cells Int. 2023 Jan 13;2023:1662182. doi: 10.1155/2023/1662182. eCollection 2023. Stem Cells Int. 2023. PMID: 39280589 Free PMC article.
References
-
- Tang X. L., Li Q., Rokosh G., et al. Long-term outcome of administration of c-kitPOS cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circulation Research. 2016;118(7):1091–1105. doi: 10.1161/CIRCRESAHA.115.307647. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

