Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies
- PMID: 30159341
- PMCID: PMC6109480
- DOI: 10.1155/2018/6984948
Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies
Abstract
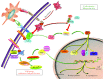
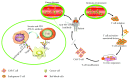
Lung cancer remains a leading cause of cancer-related mortality worldwide with the poor prognosis. Encouragingly, immune checkpoint blockade targeting programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) has dramatically changed the landscape for treatments in patients with non-small-cell lung cancer (NSCLC). However, only a small proportion of NSCLC patients responded to monotherapy of anti-PD-1/PDL1 agents; together, the development of resistance to anti-PD-1/PD-L1 therapy that leads to failure of anti-PD-1/PD-L1 therapy has significantly limited a broad applicability of the findings in clinical practices. Nowadays, several companion diagnostic assays for PDL1 expression have been introduced for identifying patients who may benefit the immunotherapy. In addition, results from clinical trials explored combinatory therapeutic strategies with conventional and/or targeted therapy reported a higher efficacy with an acceptable safety profile in NSCLC treatments, as compared to the monotherapy of these agents alone. In this review article, we summarized several anti-PD-1/PD-L1 agents licensed for NSCLC treatment, with a focus on predictive biomarkers and companion diagnostic assays for identification of NSCLC patients for immunotherapy anti-PD-1/PDL1 antibodies. Of a great interest, potentials of the combinatory therapy of anti-PD-1/PDL1 therapy with a conventional or targeted therapy, or other immunotherapy such as CAR-T cell therapy were emphasized in the article.
Figures
References
-
- Kiura K., Imamura F., Kagamu H., et al. Phase 3 study of ceritinib vs chemotherapy in ALK-rearranged NSCLC patients previously treated with chemotherapy and crizotinib (ASCEND-5): Japanese subset. Japanese Journal of Clinical Oncology. 2018;48(4):367–375. doi: 10.1093/jjco/hyy016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

