The Future of Bioorthogonal Chemistry
- PMID: 30159392
- PMCID: PMC6107859
- DOI: 10.1021/acscentsci.8b00251
The Future of Bioorthogonal Chemistry
Abstract
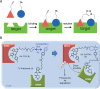
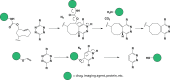
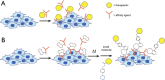
Bioorthogonal reactions have found widespread use in applications ranging from glycan engineering to in vivo imaging. Researchers have devised numerous reactions that can be predictably performed in a biological setting. Depending on the requirements of the intended application, one or more reactions from the available toolkit can be readily deployed. As an increasing number of investigators explore and apply chemical reactions in living systems, it is clear that there are a myriad of ways in which the field may advance. This article presents an outlook on the future of bioorthogonal chemistry. I discuss currently emerging opportunities and speculate on how bioorthogonal reactions might be applied in research and translational settings. I also outline hurdles that must be cleared if progress toward these goals is to be made. Given the incredible past successes of bioorthogonal chemistry and the rapid pace of innovations in the field, the future is undoubtedly very bright.
Conflict of interest statement
The author declares no competing financial interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

