The macrophage migration inhibitory factor pathway in human B cells is tightly controlled and dysregulated in multiple sclerosis
- PMID: 30160778
- PMCID: PMC6282801
- DOI: 10.1002/eji.201847623
The macrophage migration inhibitory factor pathway in human B cells is tightly controlled and dysregulated in multiple sclerosis
Abstract
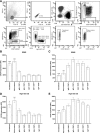
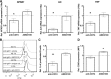
In MS, B cells survive peripheral tolerance checkpoints to mediate local inflammation, but the underlying molecular mechanisms are relatively underexplored. In mice, the MIF pathway controls B-cell development and the induction of EAE. Here, we found that MIF and MIF receptor CD74 are downregulated, while MIF receptor CXCR4 is upregulated in B cells from early onset MS patients. B cells were identified as the main immune subset in blood expressing MIF. Blocking of MIF and CD74 signaling in B cells triggered CXCR4 expression, and vice versa, with separate effects on their proinflammatory activity, proliferation, and sensitivity to Fas-mediated apoptosis. This study reveals a new reciprocal negative regulation loop between CD74 and CXCR4 in human B cells. The disturbance of this loop during MS onset provides further insights into how pathogenic B cells survive peripheral tolerance checkpoints to mediate disease activity in MS.
Keywords: Autoimmune disease; B-cell biology; Clinically isolated syndrome; MIF receptors CXCR4/CD74; MS.
© 2018 The Authors. European Journal of Immunology published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




References
-
- Hauser, S. L. , Waubant, E. , Arnold, D. L. , Vollmer, T. , Antel, J. , Fox, R. J. , Bar‐Or, A. et al., B‐cell depletion with rituximab in relapsing‐remitting multiple sclerosis. N. Engl. J. Med. 2008. 358: 676–688. - PubMed
-
- Baker, D. , O'Neill, J. K. and Turk, J. L. , Cytokines in the central nervous system of mice during chronic relapsing experimental allergic encephalomyelitis. Cell. Immunol. 1991. 134: 505–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

